推荐产品
品質等級
化驗
≥97% (HPLC)
形狀
powder
儲存條件
desiccated
顏色
white to brown
溶解度
DMSO: 20 mg/mL, clear
儲存溫度
room temp
SMILES 字串
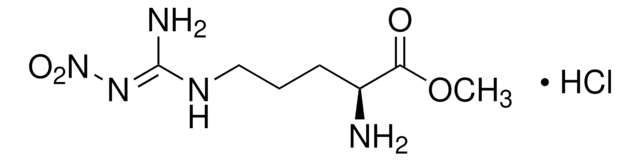
CN1C=C(C2=CC=NC=C2)C(C(C=C3)=CC=C3OCC4=NC5=CC=CC=C5C=C4)=N1.[H]Cl
InChI
1S/C25H20N4O/c1-29-16-23(18-12-14-26-15-13-18)25(28-29)20-7-10-22(11-8-20)30-17-21-9-6-19-4-2-3-5-24(19)27-21/h2-16H,17H2,1H3
InChI 密鑰
AZEXWHKOMMASPA-UHFFFAOYSA-N
生化/生理作用
PF-2545920 hydrochloride (MP-10) is a potent and selective cyclic nucleotide phosphodiesterase (PDE) 10A competitive inhibitor with a reported IC50 value of 1.26 nM. PDE10A hydrolyzes both cAMP and cGMP, and is highly expressed in medium spiny neurons of the mammalian striatum and in basal ganglia areas where D1 and D2 dopamine receptors are expressed. PF-2545920 has been studied for treatment of schizophrenia and Huntington′s disease.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Jonathan M Wilson et al.
Neuropharmacology, 99, 379-386 (2015-08-11)
Studies described here tested the hypothesis that phosphodiesterase 10A inhibition by a selective antagonist, MP-10, activates the dopamine D2 receptor expressing medium spiny neurons to a greater extent than the D1 receptor expressing neurons. We used regional pattern of c-Fos
Vahri Beaumont et al.
Neuron, 92(6), 1220-1237 (2016-12-06)
Huntington's disease (HD) symptoms are driven to a large extent by dysfunction of the basal ganglia circuitry. HD patients exhibit reduced striatal phoshodiesterase 10 (PDE10) levels. Using HD mouse models that exhibit reduced PDE10, we demonstrate the benefit of pharmacologic PDE10 inhibition
Patrick R Verhoest et al.
Journal of medicinal chemistry, 52(16), 5188-5196 (2009-07-28)
By utilizing structure-based drug design (SBDD) knowledge, a novel class of phosphodiesterase (PDE) 10A inhibitors was identified. The structure-based drug design efforts identified a unique "selectivity pocket" for PDE10A inhibitors, and interactions within this pocket allowed the design of highly
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门