推荐产品
生物源
synthetic
化驗
≥98% (TLC)
形狀
powder
技術
thin layer chromatography (TLC): suitable
顏色
white
溶解度
water: 50 mg/mL, clear, colorless
儲存溫度
−20°C
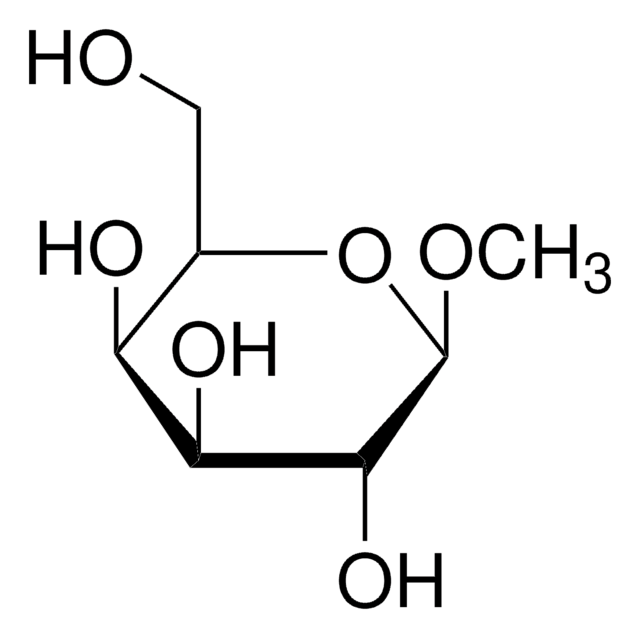
SMILES 字串
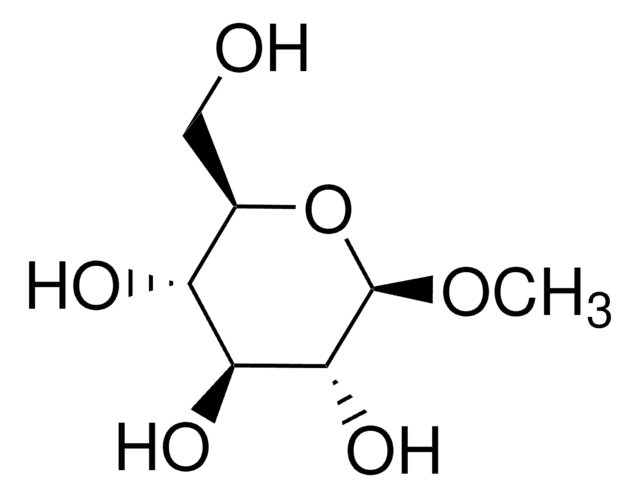
CS[C@@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O
InChI
1S/C7H14O5S/c1-13-7-6(11)5(10)4(9)3(2-8)12-7/h3-11H,2H2,1H3/t3-,4+,5+,6-,7+/m1/s1
InChI 密鑰
LZFNFLTVAMOOPJ-PZRMXXKTSA-N
正在寻找类似产品? 访问 产品对比指南
相关类别
一般說明
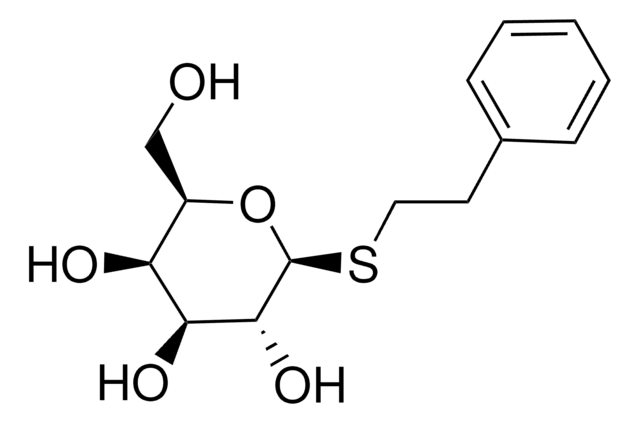
The uptake of methyl-β-D-thiogalactoside (TMG), lactose, and glucose is maintained by the phosphoenolpyruvate-dependent phosphotransferase system.
應用
Methyl-β-D-thiogalactoside has been used in a study to analyze inducers of the E.coli lac repressor system. It has also been used in a study that investigated the utilization of lactose by Streptococcus faecalis.
包裝
Bottomless glass bottle. Contents are inside inserted fused cone.
其他說明
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Christopher T Oberg et al.
Journal of medicinal chemistry, 51(7), 2297-2301 (2008-03-06)
Anionic O2 derivatives of methyl 3-deoxy-3-(4-methylbenzamido)-1-thio-beta-D-galactopyranoside have been synthesized as inhibitors against galectin-3. The sulfate, H-phosphonate, and benzyl phosphate derivatives showed an increased affinity as compared to the parent unsubstituted galactopyranoside. Modeling revealed arginine-144 being pinched by the C3 benzamide
D L Wyborski et al.
Nucleic acids research, 19(17), 4647-4653 (1991-09-11)
Although the inducible prokaryotic lac repressor system has been successfully adapted for control of gene expression in mammalian cells, little information is available on the pharmacokinetics of beta-galactoside inducers in mammalian cells for optimizing this system. These studies directly measure
β-D-phosphogalactoside galactohydrolase of Streptococcus faecalis and the inhibition of its synthesis by glucose
Heller, K. and R. Roschenthaler
Canadian Journal of Microbiology, 24, 512-519 (1979)
E Mileykovskaya et al.
Journal of bacteriology, 179(4), 1029-1034 (1997-02-01)
The CpxA-CpxR two-component signal transduction pathway of Escherichia coli was studied in a mutant (pss-93) lacking phosphatidylethanolamine (PE). Several properties of this mutant are comparable to phenotypes of cpxA point mutants, indicating that this two-component pathway is activated in PE-deficient
Anja Marbach et al.
Journal of biotechnology, 157(1), 82-88 (2011-11-15)
Most commonly used expression systems in bacteria are based on the Escherichia coli lac promoter. Furthermore, lac operon elements are used today in systems and synthetic biology. In the majority of the cases the gratuitous inducers IPTG or TMG are
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门