推荐产品
mp
96-99 °C (lit.)
儲存溫度
2-8°C
SMILES 字串
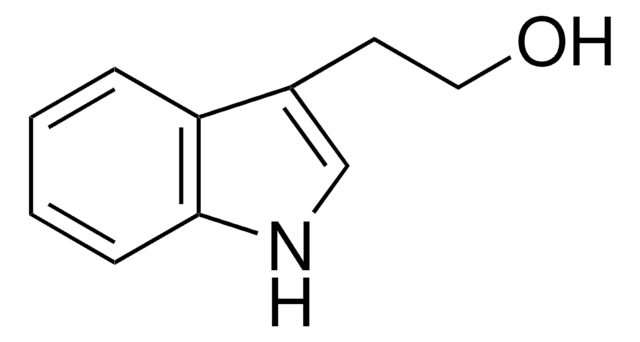
OCc1c[nH]c2ccccc12
InChI
1S/C9H9NO/c11-6-7-5-10-9-4-2-1-3-8(7)9/h1-5,10-11H,6H2
InChI 密鑰
IVYPNXXAYMYVSP-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
吲哚-3-甲醇是十字花科蔬菜中产生的一种新的次生植物代谢产物,例如卷心菜、花椰菜和抱子甘蓝等。
應用
吲哚-3-甲醇用于封装聚-乳酸-co-乙醇酸 (PLGA),以研究其对乳腺腺癌上皮细胞(MCF7)、结肠腺癌上皮细胞(Caco2)、前列腺癌上皮细胞(PC3)的体外抗癌效果。它还被用作细胞色素P4501A (CYP1A)诱导物。
生化/生理作用
吲哚-3-甲醇(I3C)激活芳烃受体(AhR),诱导G1细胞周期阻滞和凋亡。因此,它是一种潜在的抗癌剂。此外,它通过刺激细胞色素P450酶来诱导雌二醇代谢。因此,I3C被认为是一种有效的化疗药物,用于各种类型的癌症,包括乳腺癌、前列腺癌、结肠癌和白血病。
在起始阶段抑制致癌症发生。已经证明在几种动物中具有抑制癌症发生的作用,但如果在发病后阶段给药,它会增加肿瘤的发病率。 在十字花科蔬菜中发现。
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Aromatic hydrocarbon responsiveness-receptor agonists generated from indole-3-carbinol in vitro and in vivo: comparisons with 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin.
Bjeldanes LF, et al.
Proceedings of the National Academy of Sciences of the USA, 88(21), 9543-9547 (1991)
Jing-Ru Weng et al.
Cancer letters, 262(2), 153-163 (2008-03-04)
During the course of oncogenesis and tumor progression, cancer cells constitutively upregulate signaling pathways relevant to cell proliferation and survival as a strategy to overcome genomic instability and acquire resistance phenotype to chemotherapeutic agents. In light of this clinical and
G S Bailey et al.
Journal of the National Cancer Institute, 78(5), 931-934 (1987-05-01)
Indole-3-carbinol (I3C), a natural constituent of cruciferous vegetables, is an inhibitor in several experimental animal models of carcinogenesis by polynuclear aromatic hydrocarbons or aflatoxin B1 (AFB1) when administered prior to or during carcinogen exposure. For assessment of the postinitiation effects
H L Bradlow et al.
Annals of the New York Academy of Sciences, 889, 204-213 (2000-02-11)
Previous studies from this laboratory have suggested that 2-hydroxyestrone is protective against breast cancer, whereas the other principal metabolite, 16 alpha-hydroxyestrone, and the lesser metabolite quantitatively, 4-hydroxyestrone, are potent carcinogens. Attempts to directly decrease the formation of the 16-hydroxylated metabolite
PLGA encapsulation and radioiodination of indole-3-carbinol: investigation of anticancerogenic effects against MCF7, Caco2 and PC3 cells by in vitro assays
Yildiz G, et al.
J. Radioanal. Nucl. Chem., 311(2), 1043-1052 (2017)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门