推荐产品
化驗
≥98% (HPLC)
應用
metabolomics
vitamins, nutraceuticals, and natural products
儲存溫度
−20°C
SMILES 字串
CCCCCCCCC[C@H](O)CC(=O)CCc1ccc(O)c(OC)c1
InChI
1S/C21H34O4/c1-3-4-5-6-7-8-9-10-18(22)16-19(23)13-11-17-12-14-20(24)21(15-17)25-2/h12,14-15,18,22,24H,3-11,13,16H2,1-2H3/t18-/m0/s1
InChI 密鑰
AIULWNKTYPZYAN-SFHVURJKSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
[10]-Gingerol is a pungent, non-volatile phenolic compound of fresh ginger root.
應用
[10]-Gingerol has been used as an antibacterial agent to study its effects on Escherichia coli ATP synthase. It has also been used as an anti-hyperglycemic agent to test its effects on promoting glucose utilization in 3T3-L1 adipocytes and C2C12 myotubes.
生化/生理作用
[10]-Gingerol is a potent anti-cancer agent and a known inhibitor of breast cancer cells growth by blocking cell proliferation and inducing programmed cell death. It also inhibits ovarian cancer cells growth by inducing G2 phase cell cycle arrest. 10-Gingerol elicits inhibitory effects towards triple breast cancer cells in both mouse models and in vitro studies. It also displays anti-neuroinflammatory and anti-hyperglycemic activities.
[10]-Gingerol is a bioactive compound found in ginger (Zingiber officinale) with anti-inflammatory and antioxidant activity.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
331.3 °F - closed cup
閃點(°C)
166.3 °C - closed cup
其他客户在看
Andrea Rasmussen et al.
Advanced pharmaceutical bulletin, 9(4), 685-689 (2019-12-21)
Purpose: Gingerol homologs found in the rhizomes of ginger plants have the potential to benefit human health, including the prevention and treatment of cancer. This study evaluated the effect of 10-gingerol on ovarian cancer cell (HEY, OVCAR3, and SKOV-3) growth.
Megan M Bernard et al.
Experimental and molecular pathology, 102(2), 370-376 (2017-03-21)
The ginger rhizome is rich in bioactive compounds, including [6]-gingerol, [8]-gingerol, and [10]-gingerol; however, to date, most research on the anti-cancer activities of gingerols have focused on [6]-gingerol. In this study, we compared [10]-gingerol with [8]-gingerol and [6]-gingerol in terms
Zhufeng Wu et al.
The Journal of pharmacy and pharmacology, 67(4), 583-596 (2014-12-17)
To determine the reaction kinetics for regioselective glucuronidation of gingerols (i.e. 6-, 8- and 10-gingerol) by human liver microsomes and expressed UDP-glucuronosyltransferase (UGT) enzymes, and to identify the main UGT enzymes involved in regioselective glucuronidation of gingerols. The rates of
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门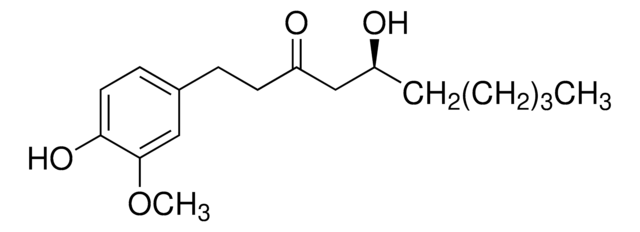
![[10]-姜酮醇 analytical standard](/deepweb/assets/sigmaaldrich/product/structures/224/210/b4f3e699-03b9-4112-89c1-a63f196344d0/640/b4f3e699-03b9-4112-89c1-a63f196344d0.png)

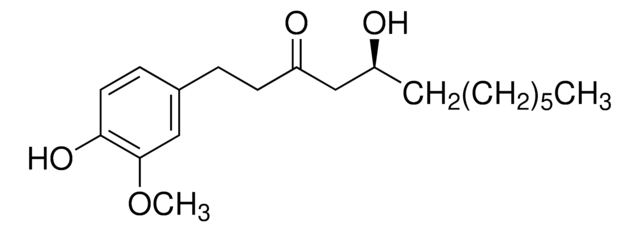
![[6]-姜烯酚 analytical standard](/deepweb/assets/sigmaaldrich/product/structures/378/737/6e6bd2f9-0152-45e1-8006-344ca92937be/640/6e6bd2f9-0152-45e1-8006-344ca92937be.png)



![[6]-Gingerol, Zingiber officinale An antitumor, apoptosis-inducing compound of the ginger family that blocks EGF-induced cell transformation by inhibiting the activation of Activator Protein-1 (AP-1).](/deepweb/assets/sigmaaldrich/product/images/140/919/0846df46-0b67-4c28-b99d-87e177be65b2/640/0846df46-0b67-4c28-b99d-87e177be65b2.jpg)
