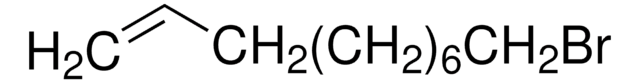推荐产品
生物源
Castanospermum australe seeds
產品線
BioUltra
化驗
≥94% (GC)
形狀
powder
mp
212-215 °C (dec.)
溶解度
1 M HCl: 20 mg/mL, clear, colorless to faintly yellow
儲存溫度
2-8°C
SMILES 字串
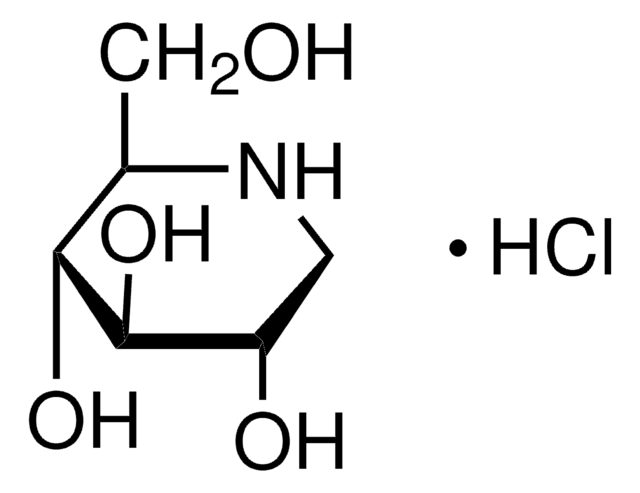
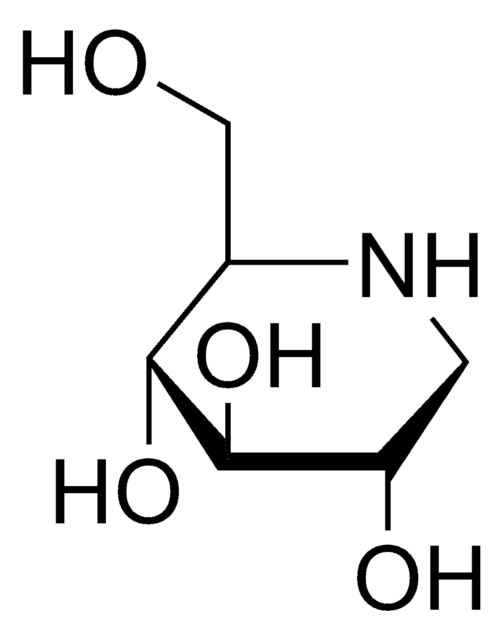
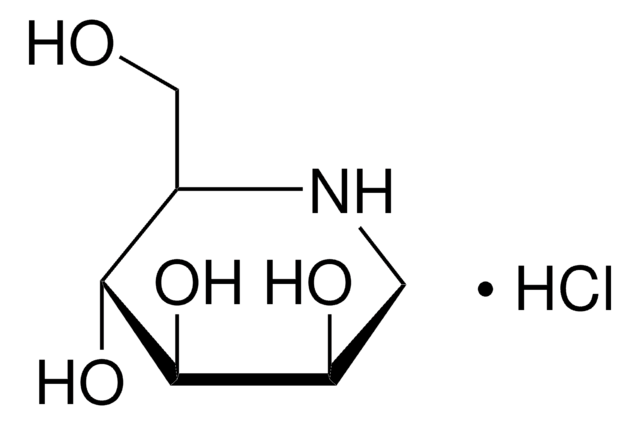
O[C@H]1CCN2C[C@H](O)[C@@H](O)[C@H](O)[C@@H]12
InChI
1S/C8H15NO4/c10-4-1-2-9-3-5(11)7(12)8(13)6(4)9/h4-8,10-13H,1-3H2/t4-,5-,6+,7+,8+/m0/s1
InChI 密鑰
JDVVGAQPNNXQDW-TVNFTVLESA-N
正在寻找类似产品? 访问 产品对比指南
生化/生理作用
α-glucosidase Inhibitor
儲存類別代碼
13 - Non Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
M R Bartlett et al.
Immunology and cell biology, 72(5), 367-374 (1994-10-01)
The glycoprotein processing inhibitor castanospermine (CS) and the monosaccharide mannose-6-phosphate (M6P), as well as some sulfated polysaccharides (SPS), have been shown to inhibit inflammation in rat models of experimental autoimmune encephalomyelitis and adjuvant-induced arthritis. Here, the anti-inflammatory effects of these
K S Ajish Kumar et al.
Organic & biomolecular chemistry, 6(4), 703-711 (2008-02-12)
The utility of a D-glucose-derived aziridine carboxylate was demonstrated for the synthesis of polyhydroxylated quinolizidine and indolizidine alkaloids. The chemoselective reduction of 1 followed by two-carbon homologation by the Wittig reaction afforded gamma,delta-aziridino-alpha,beta-unsaturated ester 9, which on regioselective nucleophilic aziridine
Julien Ceccon et al.
Organic letters, 8(21), 4739-4742 (2006-10-06)
[reaction: see text] An asymmetric total synthesis of (-)-swainsonine and (+)-6-epicastanospermine is described from a common intermediate, which is obtained through diastereoselective [2 + 2] cycloaddition of dichloroketene to a chiral enol ether.
Satoru Watanabe et al.
Antiviral research, 96(1), 32-35 (2012-08-08)
Celgosivir (6-O-butanoyl castanospermine), a pro-drug of the naturally occurring castanospermine, is an inhibitor of α-glucosidase I and II that is found to be a potent inhibitor of several enveloped viruses including all four serotypes of dengue virus. We showed previously
Vinod P Vyavahare et al.
Journal of medicinal chemistry, 50(22), 5519-5523 (2007-10-09)
Two new C-1 epimeric hydroxymethyl castanospermine congeners 2a and 2b, synthesized by stereocontrolled intramolecular double reductive amination of D-glucose derived beta-keto ester as a key step, showed impressive immuno-potentiating property. The bioactivity was mediated through up-regulation of T(H1)/T(H2) cytokine ratio.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门