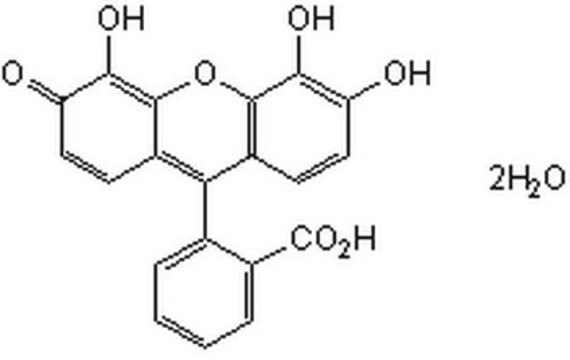
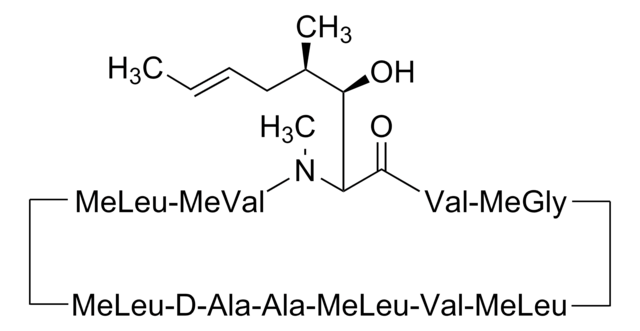
推荐产品
product name
环孢菌素A, from Tolypocladium inflatum, ≥95% (HPLC), solid
生物源
Tolypocladium inflatum
品質等級
化驗
≥95% (HPLC)
形狀
solid
顏色
white
溶解度
dichloromethane: 10 mg/mL
ethanol: 10 mg/mL
DMSO: 50 mg/mL
chloroform: 6 mg/mL
H2O: insoluble
抗生素活性譜
fungi
作用方式
enzyme | inhibits
起源
Novartis
儲存溫度
2-8°C
SMILES 字串
CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C
InChI
1S/C62H111N11O12/c1-25-27-28-40(15)52(75)51-56(79)65-43(26-2)58(81)67(18)33-48(74)68(19)44(29-34(3)4)55(78)66-49(38(11)12)61(84)69(20)45(30-35(5)6)54(77)63-41(16)53(76)64-42(17)57(80)70(21)46(31-36(7)8)59(82)71(22)47(32-37(9)10)60(83)72(23)50(39(13)14)62(85)73(51)24/h25,27,34-47,49-52,75H,26,28-33H2,1-24H3,(H,63,77)(H,64,76)(H,65,79)(H,66,78)/b27-25+/t40-,41+,42-,43+,44+,45+,46+,47+,49+,50+,51+,52-/m1/s1
InChI 密鑰
PMATZTZNYRCHOR-CGLBZJNRSA-N
基因資訊
human ... PPIA(5478) , PPP3CA(5530) , PPP3CB(5532) , PPP3CC(5533) , PPP3R1(5534) , PPP3R2(5535)
正在寻找类似产品? 访问 产品对比指南
生化/生理作用
特點和優勢
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 4 Oral - Carc. 1B - Repr. 1B
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
個人防護裝備
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
其他客户在看
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门