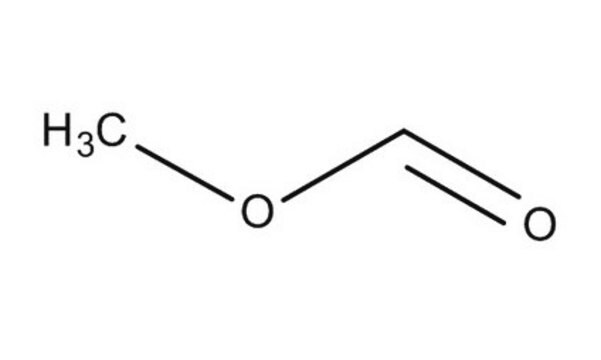
推荐产品
等級
spectrophotometric grade
品質等級
蒸汽密度
2.1 (vs air)
蒸汽壓力
32.81 psi ( 55 °C)
9.21 psi ( 20 °C)
化驗
≥98%
形狀
liquid
自燃溫度
842 °F
expl. lim.
23 %
技術
UV/Vis spectroscopy: suitable
雜質
<0.010% water
蒸發殘留物
<0.0005%
折射率
n20/D 1.343 (lit.)
pH值
4.0-5.0 (20 °C, 200 g/L)
bp
32-34 °C (lit.)
mp
−100 °C (lit.)
密度
0.974 g/mL at 20 °C (lit.)
&lambda ;
H2O reference
紫外吸收
λ: 259 nm Amax: 1.00
λ: 260 nm Amax: 0.70
λ: 265 nm Amax: 0.20
λ: 270 nm Amax: 0.04
λ: 310-400 nm Amax: 0.01
SMILES 字串
[H]C(=O)OC
InChI
1S/C2H4O2/c1-4-2-3/h2H,1H3
InChI 密鑰
TZIHFWKZFHZASV-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
Methyl formate (MF) is one of the most significant intermediates in C1 chemistry. MF has been used to produce methanamide, N, N-dimethylformamide, dimethyl carbonate, methyl glycolate, acetic acid, ethylene glycol, methyl methacrylate, and high-purity CO. Additionally, MF can be used as a solvent for nitrocellulose and cellulose acetate.
應用
- Bifunctionality of Re Supported on TiO(2) in Driving Methanol Formation in Low-Temperature CO(2) Hydrogenation.: This article explores the catalytic roles of methyl formate in methanol production from CO2 hydrogenation, providing a pathway for sustainable chemical synthesis (Urakawa A et al., 2023).
訊號詞
Danger
危險分類
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Eye Irrit. 2 - Flam. Liq. 1 - STOT SE 1 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 2
閃點(°F)
-2.2 °F - closed cup
閃點(°C)
-19 °C - closed cup
Katherine R Phillips et al.
Journal of the American Chemical Society, 135(2), 574-577 (2012-12-28)
Methyl formate is produced from the photo-oxidation of methanol on preoxidized TiO(2)(110). We demonstrate that two consecutive photo-oxidation steps lead to methyl formate using mass spectrometry and scanning tunneling microscopy. The first step in methanol oxidation is formation of methoxy
Methyl N-Phenyl Carbamate Synthesis from Aniline and Methyl Formate: Carbon Recycling to Chemical Products.
Yalfani MS, et al.
ChemSusChem, 8(3), 443-447 (2015)
Methyl formate as a carbonylating agent for the catalytic conversion of phenol to methyl phenyl carbonate.
Wu C, et al.
Green Chemistry, 17(3), 1467-1472 (2015)
Barriers to rotation adjacent to double bonds. 3. The carbon-oxygen barrier in formic acid, methyl formate, acetic acid, and methyl acetate. The origin of ester and amide resonance.
Wiberg KB and Laidig KE.
Journal of the American Chemical Society, 109(20), 5935-5943 (1987)
Microwave spectrum, barrier to internal rotation, and structure of methyl formate.
Curl Jr RF.
J. Chem. Phys. , 30(6), 1529-1536 (1959)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门