推荐产品
等級
pharmaceutical primary standard
API 家族
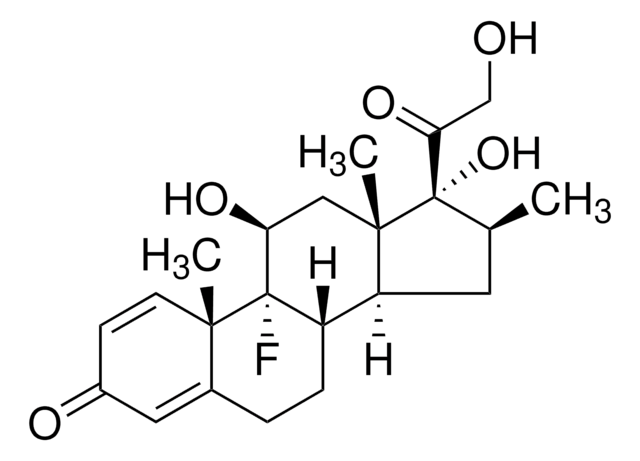
betamethasone
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
2-8°C
SMILES 字串
CCCCC(=O)O[C@@]1([C@@H](C)CC2C3CCC4=CC(=O)C=C[C@]4(C)[C@@]3(F)[C@@H](O)C[C@]12C)C(=O)CO
InChI
1S/C27H37FO6/c1-5-6-7-23(33)34-27(22(32)15-29)16(2)12-20-19-9-8-17-13-18(30)10-11-24(17,3)26(19,28)21(31)14-25(20,27)4/h10-11,13,16,19-21,29,31H,5-9,12,14-15H2,1-4H3/t16-,19?,20?,21-,24-,25-,26-,27-/m0/s1
InChI 密鑰
SNHRLVCMMWUAJD-QDHNOTTGSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Betamethasone valerate for system suitability EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
产品编号
说明
价格
訊號詞
Danger
危險聲明
危險分類
Repr. 1B - STOT RE 2
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 2
Luigi Naldi et al.
American journal of clinical dermatology, 12(3), 191-201 (2011-02-03)
Corticosteroids are a versatile option for the treatment of mild-to-moderate psoriasis due to their availability in a wide range of potencies and formulations. Occlusion of the corticosteroid is a widely accepted procedure to enhance the penetration of the medication, thereby
Jin Zhang et al.
Journal of pharmaceutical sciences, 100(3), 896-903 (2010-09-15)
Corticosteroids are therapeutic agents widely used in the pharmacological treatment of skin diseases such as eczema or psoriasis. Unfortunately, their use is restricted by the side effects that frequently occur at the systemic level. The goal of the research described
Jayalakshmi Somuramasami et al.
Journal of pharmaceutical and biomedical analysis, 54(1), 242-247 (2010-09-11)
Currently, there are no analytical methods available in the literature that can simultaneously separate and quantitate residual levels of acetone, methylene chloride, n-butyl ether and dimethylsulfoxide in Betamethasone valerate active pharmaceutical ingredient (API). This paper describes the development and validation
An occlusive dressing containing betamethasone valerate 0.1% for the treatment of prurigo nodularis.
Rosita Saraceno et al.
The Journal of dermatological treatment, 21(6), 363-366 (2010-06-12)
Prurigo nodularis is a distressing condition characterized by the presence of multiple nodules associated with intense pruritus. To assess the clinical efficacy and safety of betamethasone valerate 0.1% tape and a moisturizing itch-relief cream in prurigo nodularis. Twelve patients were
Paiboon Sookpotarom et al.
Pediatric surgery international, 29(4), 393-396 (2013-01-08)
0.05 % betamethasone valerate cream is generally used as an alternative to circumcision for the treatment of phimosis in boys. The aim of this study is to determine whether the half-strength formula (0.025 %) of betamethasone is as effective as 0.05 % betamethasone.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门