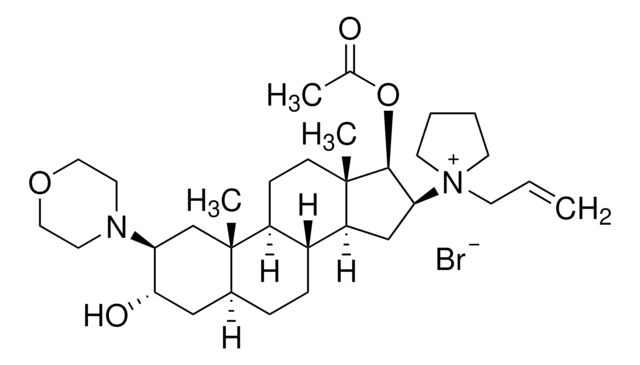
About This Item
推荐产品
等級
pharmaceutical primary standard
API 家族
rocuronium
製造商/商標名
EDQM
溶解度
H2O: 100 mg/mL, clear, light yellow
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
−20°C
SMILES 字串
[Br-].CC(=O)O[C@H]1[C@H](C[C@H]2[C@@H]3CC[C@H]4C[C@H](O)[C@H](C[C@]4(C)[C@H]3CC[C@]12C)N5CCOCC5)[N+]6(CCCC6)CC=C
InChI
1S/C32H53N2O4.BrH/c1-5-14-34(15-6-7-16-34)28-20-26-24-9-8-23-19-29(36)27(33-12-17-37-18-13-33)21-32(23,4)25(24)10-11-31(26,3)30(28)38-22(2)35;/h5,23-30,36H,1,6-21H2,2-4H3;1H/q+1;/p-1/t23-,24+,25-,26-,27-,28-,29-,30-,31-,32-;/m0./s1
InChI 密鑰
OYTJKRAYGYRUJK-FMCCZJBLSA-M
基因資訊
human ... CHRNA1(1134) , CHRNB1(1140) , CHRND(1144) , CHRNE(1145) , CHRNG(1146)
正在寻找类似产品? 访问 产品对比指南
相关类别
一般說明
應用
生化/生理作用
包裝
其他說明
相關產品
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门