推荐产品
等級
pharmaceutical primary standard
API 家族
ketorolac
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
2-8°C
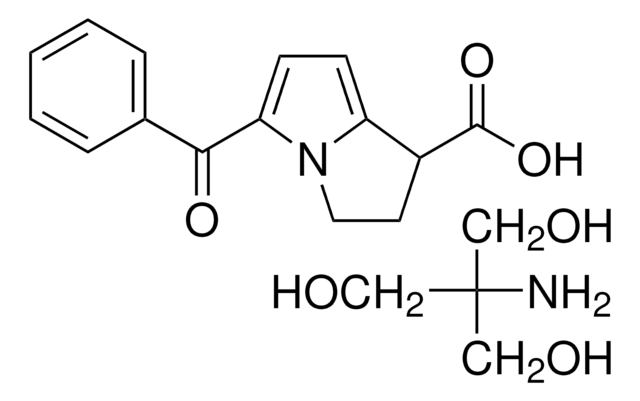
SMILES 字串
NC(CO)(CO)CO.OC(=O)C1CCn2c1ccc2C(=O)c3ccccc3
InChI
1S/C15H13NO3.C4H11NO3/c17-14(10-4-2-1-3-5-10)13-7-6-12-11(15(18)19)8-9-16(12)13;5-4(1-6,2-7)3-8/h1-7,11H,8-9H2,(H,18,19);6-8H,1-3,5H2
InChI 密鑰
BWHLPLXXIDYSNW-UHFFFAOYSA-N
基因資訊
human ... PTGS1(5742) , PTGS2(5743)
正在寻找类似产品? 访问 产品对比指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Ketorolac trometamol EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
产品编号
说明
价格
訊號詞
Danger
危險分類
Acute Tox. 3 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Michel Weber et al.
Acta ophthalmologica, 91(1), e15-e21 (2012-09-14)
To determine whether indomethacin 0.1% eye drops are at least as effective as ketorolac 0.5% eye drops in treating ocular inflammation following cataract surgery. Prospective, multicenter, investigator-masked, parallel-group, randomized, active-controlled clinical trial. Cataract patients were randomized in a 1:1 ratio
Stephen J Kim et al.
Archives of ophthalmology (Chicago, Ill. : 1960), 130(4), 456-460 (2012-04-12)
To investigate the adverse ocular effects of intravitreal ketorolac (4 mg) in patients with chronic uveitis and complications of chronic inflammation (macular edema). We conducted a prospective phase 1 clinical trial involving 10 eyes of 10 adult patients with chronic
V Pfaffenrath et al.
Cephalalgia : an international journal of headache, 32(10), 766-777 (2012-06-20)
Ketorolac is a non-triptan, non-opioid, mixed cyclooxygenase (COX)1/2-inhibitor for short-term management of moderate-to-severe acute pain. This trial evaluated an intranasal formulation of ketorolac tromethamine (SPRIX®) containing 6% lidocaine (ROX-828) for the acute treatment of migraine with and without aura as
Meizi Wang et al.
Retina (Philadelphia, Pa.), 32(10), 2158-2164 (2012-10-27)
To investigate the concentrations and pharmacokinetics of ketorolac in the rabbits by three different routes of administrations: a single intracameral, intravitreal, and suprachoroidal injection. Fifty-four New Zealand white rabbits received ketorolac (250 μg/0.05 mL) in one eye by a single
Mrinal Kanti Bain et al.
International journal of biological macromolecules, 50(3), 565-572 (2012-02-04)
The effect of molecular weight of poly(vinyl alcohol) (PVA) and sodium chloride on the gelation temperature of methylcellulose (MC) was studied with the objective to develop a MC based formulation for sustained delivery of ketorolac tromethamine a model ophthalmic drug.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门