推荐产品
等級
pharmaceutical primary standard
API 家族
tramadol
製造商/商標名
EDQM
藥物控制
USDEA Schedule IV
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
2-8°C
SMILES 字串
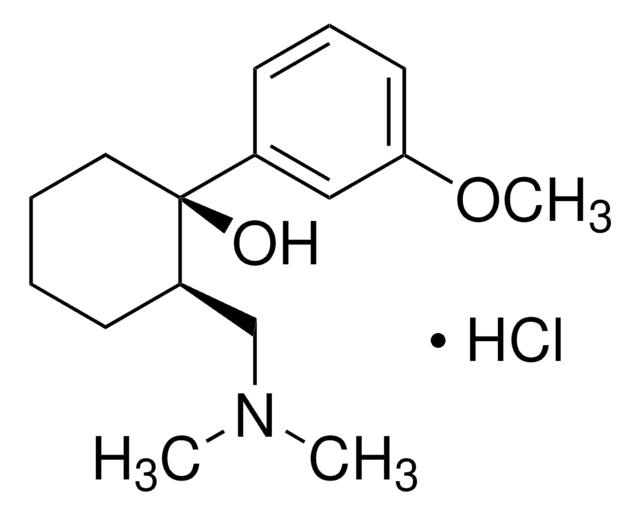
Cl.COc1cccc(c1)[C@@]2(O)CCCC[C@@H]2CN(C)C
InChI
1S/C16H25NO2.ClH/c1-17(2)12-14-7-4-5-10-16(14,18)13-8-6-9-15(11-13)19-3;/h6,8-9,11,14,18H,4-5,7,10,12H2,1-3H3;1H/t14-,16+;/m1./s1
InChI 密鑰
PPKXEPBICJTCRU-XMZRARIVSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
Tramadol is a synthetic opiate agonist analgesic, widely used in patients suffering from moderate to severe chronic pain. It is considered safe when compared to other opioids. It contributes to the analgesic activity by blocking the nociceptive impulses at the spinal level, inhibiting the norepinephrine and serotonin reuptake.
Tramadol is a synthetic opiate agonist analgesic, widely used in patients suffering from moderate to severe chronic pain. It is considered safe when compared to other opioids. It contributes to the analgesic activity by blocking the nociceptive impulses at the spinal level, inhibiting the norepinephrine and serotonin reuptake.
應用
This European Pharmacopoeia reference standard is intended for use only as specifically prescribed in the European Pharmacopoeia.
生化/生理作用
Opioid analgesic; P450 substrate.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral - Aquatic Chronic 2 - STOT SE 3
標靶器官
Central nervous system
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Analgesic oral efficacy of tramadol hydrochloride in postoperative pain
Sunshine A, et al.
Clinical Pharmacology and Therapeutics, 51(6), 740-746 (1992)
Application of HPLC for the simultaneous determination of aceclofenac, paracetamol and tramadol hydrochloride in pharmaceutical dosage form
Chandra P, et al.
Scientia Pharmaceutica, 80(2), 337-352 (2012)
Emrah Yuruk et al.
Journal of endourology, 29(4), 463-467 (2014-10-01)
To compare the outcomes of these minimally invasive procedures in this patient population. The database of our institution has been retrospectively reviewed, and medical records of urolithiasis patients with a solitary kidney who underwent flexible ureteroscopy (F-URS) or extracorporeal shock
Guojun Wu et al.
International journal of clinical and experimental pathology, 7(9), 5665-5673 (2014-10-23)
This study is to establish the rhesus monkey model of lymphedema in the upper limbs, and assess the suitability of this model. An animal model of lymphedema was established by the combined irradiation and surgical techniques in the upper limbs
Marco Cespi et al.
International journal of pharmaceutics, 477(1-2), 140-147 (2014-10-12)
The use of process analytical technologies (PAT) to ensure final product quality is by now a well established practice in pharmaceutical industry. To date, most of the efforts in this field have focused on development of analytical methods using spectroscopic
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门