推荐产品
等級
certified reference material
pharmaceutical secondary standard
品質等級
agency
traceable to BP 1173
traceable to Ph. Eur. Y0001405
traceable to USP 1478367
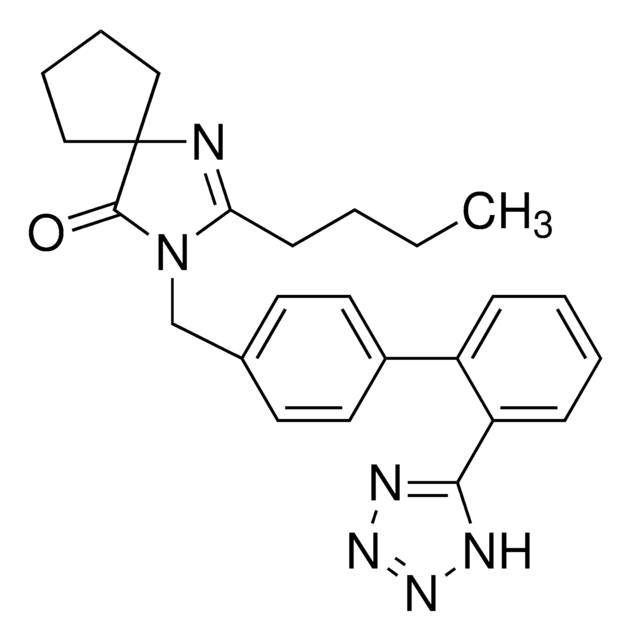
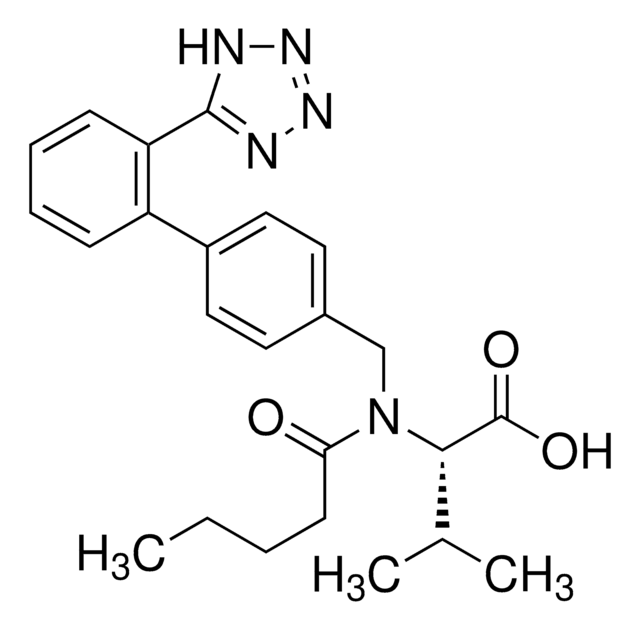
API 家族
olmesartan
CofA
current certificate can be downloaded
包裝
pkg of 200 mg
應用
pharmaceutical
形式
neat
儲存溫度
-10 to -25°C
SMILES 字串
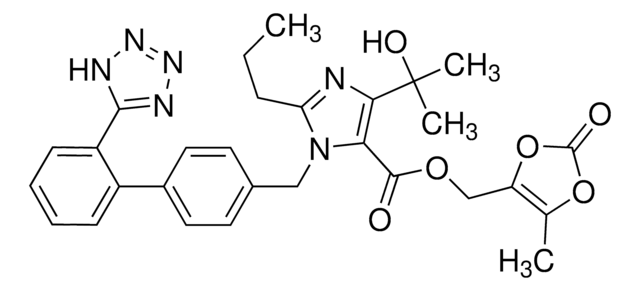
CCCC1=NC(C(O)(C)C)=C(C(OCC2=C(C)OC(O2)=O)=O)N1CC(C=C3)=CC=C3C4=CC=CC=C4C5=NN=NN5
InChI
1S/C29H30N6O6/c1-5-8-23-30-25(29(3,4)38)24(27(36)39-16-22-17(2)40-28(37)41-22)35(23)15-18-11-13-19(14-12-18)20-9-6-7-10-21(20)26-31-33-34-32-26/h6-7,9-14,38H,5,8,15-16H2,1-4H3,(H,31,32,33,34)
InChI 密鑰
UQGKUQLKSCSZGY-UHFFFAOYSA-N
基因資訊
human ... AGTR1(185)
正在寻找类似产品? 访问 产品对比指南
一般說明
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Olmesartan Medoxomil is an AT1 subtype angiotensin-II receptor antagonist used in the management of hypertension. It prevents angiotensin II from binding to the AT1 receptors, thereby decreasing vasoconstriction.
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Olmesartan Medoxomil is an AT1 subtype angiotensin-II receptor antagonist used in the management of hypertension. It prevents angiotensin II from binding to the AT1 receptors, thereby decreasing vasoconstriction.
應用
Theis pharmaceutical secondary standard can also be used as follows:
- Development and validation of an isocratic ultra-high performance liquid chromatography (UHPLC) based stability indicating method to determine olmesartan medoxomil and amlodipine besylate in combined tablet dosage forms
- Simultaneous determination of Olmesartan medoxomil and chlorthalidone by reversed phase-high performance liquid chromatography (RP-HPLC) in tablets
- Quantification of olmesartan medoxomil and its degradation products in bulk drugs and pharmaceutical formulations by using a systematic quality by design (QbD)-based reverse-phase liquid chromatography method
- Reversed phase-high performance liquid chromatographic (RP-HPLC) analysis of olmesartan medoxomil and hydrochlorothiazide in their combined tablet dosage form
- Determination of olmesartan medoxomil and amlodipine besylate in their combined tablet dosage form by absorption subtraction method, ratio subtraction with extended ratio subtraction method, dual wavelength technique, and second order derivative spectrophotometry
分析報告
These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.
腳註
To see an example of a Certificate of Analysis for this material enter LRAC2529 in the Documents slot below. This is an example certificate only and may not be the lot that you receive.
相關產品
产品编号
说明
价格
訊號詞
Warning
危險分類
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Development and validation of RP-HPLC method for the simulteneous estimation of olmesartan medoxomil and chlorthalidone in tablet dosage form
Sawale V, et al.
International Journal of Pharmacy and Pharmaceutical Sciences, 7, 266-269 (2015)
Development and validation of RP-HPLC method for simultaneous determination of a combined formulation of olmesartan medoxomil & hydrochlorothiazide
Sony A, et al.
World Journal of Pharmacy and Pharmaceutical Sciences, 9, 1468-1488 (2020)
DAD based stability indicating RP-UPLC method for simultaneous determination of olmesartan medoxomil and amlodipine besylate
Kerai JR, et al.
Pharmaceutical Chemistry Journal, 52, 959-964 (2019)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门