推荐产品
agency
USP/NF
meets USP testing specifications
品質等級
形狀
solid
溶解度
H2O: 50 mg/mL
抗生素活性譜
Gram-negative bacteria
Gram-positive bacteria
應用
pharmaceutical (small molecule)
作用方式
cell wall synthesis | interferes
儲存溫度
2-8°C
SMILES 字串
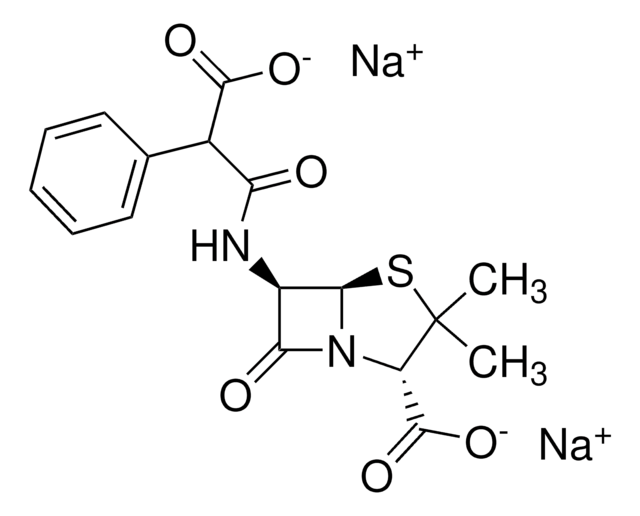
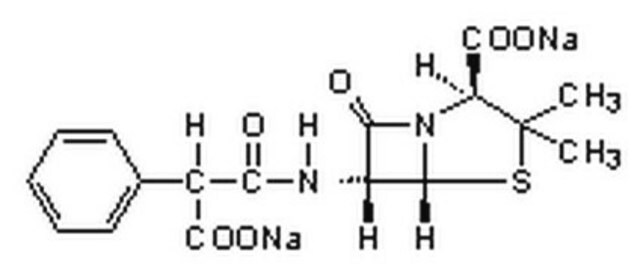
[Na+].[Na+].CC1(C)S[C@@H]2[C@H](NC(=O)C(C([O-])=O)c3ccccc3)C(=O)N2[C@H]1C([O-])=O
InChI
1S/C17H18N2O6S.2Na/c1-17(2)11(16(24)25)19-13(21)10(14(19)26-17)18-12(20)9(15(22)23)8-6-4-3-5-7-8;;/h3-7,9-11,14H,1-2H3,(H,18,20)(H,22,23)(H,24,25);;/q;2*+1/p-2/t9?,10-,11+,14-;;/m1../s1
InChI 密鑰
RTYJTGSCYUUYAL-YCAHSCEMSA-L
正在寻找类似产品? 访问 产品对比指南
一般說明
Chemical structure: ß-lactam
應用
Used for selection of ampr transformed cells. Used to study the role of penicillin-sensitive transpeptidases in cell wall biosynthesis.
生化/生理作用
作用机制:羧青霉素抗生素,可通过灭活细菌细胞膜内表面的转肽酶来抑制细菌细胞壁合成(肽聚糖交联)。
抗微生物谱:具有抗革兰氏阳性和革兰氏阴性细菌活性。
抗微生物谱:具有抗革兰氏阳性和革兰氏阴性细菌活性。
分析報告
在 37°C 下可稳定保存 3 天
其他說明
Keep container tightly closed in a dry and well-ventilated place.
訊號詞
Danger
危險聲明
危險分類
Resp. Sens. 1 - Skin Sens. 1
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
历史批次信息供参考:
分析证书(COA)
Lot/Batch Number
其他客户在看
Olivier Fisette et al.
Biophysical journal, 103(8), 1790-1801 (2012-10-23)
The effects of substrate binding on class A β-lactamase dynamics were studied using molecular dynamics simulations of two model enzymes; 40 100-ns trajectories of the free and substrate-bound forms of TEM-1 (with benzylpenicillin) and PSE-4 (with carbenicillin) were recorded (totaling
Nina Möker et al.
Journal of bacteriology, 192(7), 1946-1955 (2010-01-26)
Bacterial persister cells constitute a small portion of a culture which is tolerant to killing by lethal doses of bactericidal antibiotics. These phenotypic variants are formed in numerous bacterial species, including those with clinical relevance like the opportunistic pathogen Pseudomonas
John R Zupan et al.
Proceedings of the National Academy of Sciences of the United States of America, 110(22), 9060-9065 (2013-05-16)
Growth and cell division in rod-shaped bacteria have been primarily studied in species that grow predominantly by peptidoglycan (PG) synthesis along the length of the cell. Rhizobiales species, however, predominantly grow by PG synthesis at a single pole. Here we
Sarah Sainsbury et al.
Journal of molecular biology, 405(1), 173-184 (2010-10-27)
We report the first crystal structures of a penicillin-binding protein (PBP), PBP3, from Pseudomonas aeruginosa in native form and covalently linked to two important β-lactam antibiotics, carbenicillin and ceftazidime. Overall, the structures of apo and acyl complexes are very similar;
Edward Avilés et al.
Organic letters, 12(22), 5290-5293 (2010-10-23)
Monamphilectine A (1), a new diterpenoid β-lactam alkaloid showing potent antimalarial activity, was isolated in milligram quantities following bioassay-directed extraction of a Puerto Rican marine sponge Hymeniacidon sp. Its structure, established by interpretation of spectral data, was confirmed unequivocally by
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门