推荐产品
等級
analytical standard
品質等級
技術
HPLC: suitable
gas chromatography (GC): suitable
應用
forensics and toxicology
pharmaceutical (small molecule)
veterinary
形式
neat
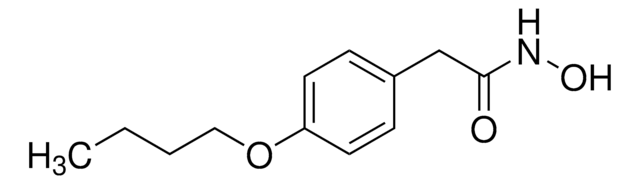
SMILES 字串
CCCCOc1ccc(CC(=O)NO)cc1
InChI
1S/C12H17NO3/c1-2-3-8-16-11-6-4-10(5-7-11)9-12(14)13-15/h4-7,15H,2-3,8-9H2,1H3,(H,13,14)
InChI 密鑰
MXJWRABVEGLYDG-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
Bufexamac is a drug with antipyretic, analgesic and anti-inflammatory properties.
應用
Bufexamac may be used as a reference standard in the determination of bufexamac in pharmaceutical formulations using high-performance liquid chromatography (HPLC).
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
推薦產品
Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
P H Marttinen et al.
Journal of veterinary medicine. A, Physiology, pathology, clinical medicine, 53(6), 311-318 (2006-08-12)
The objective here was to evaluate the acute effects of induced arthritis on synovial fluid (SF) levels of matrix metalloproteinases (MMP) -2, -8 and -9 in horses. To evaluate MMP-2 and -9 activities and the effect of non-steroidal anti-inflammatory drug
Ritu Gupta et al.
The Australasian journal of dermatology, 47(2), 117-119 (2006-04-28)
A 24-year-old woman had a 9-week history of second to third daily urticaria that began after an episode of contact urticaria to topical bufexamac. She was found to have an underlying gastrointestinal infection with Blastocystis hominis. This was thought to
K Waltermann et al.
Der Hautarzt; Zeitschrift fur Dermatologie, Venerologie, und verwandte Gebiete, 60(5), 424-427 (2008-12-19)
We report on a case of a bufexamac-induced allergic contact dermatitis with hematogenous dissemination presenting with the clinical and histological picture of a pigmented purpuric eruption. To our knowledge this is the first report on a bufexamac-induced pigmented purpuric dermatosis.
Hagen Trommer et al.
The Journal of pharmacy and pharmacology, 55(10), 1379-1388 (2003-11-11)
The effect of bufexamac on UV-irradiation-induced lipid peroxidation was investigated. Linolenic acid was used as a model lipid. Bufexamac was shown to be capable of reducing the amount of lipid peroxidation. The quantification was carried out by the thiobarbituric acid
Contact sensitivity to bufexamac.
Lachapelle JM.
Contact Dermatitis, 1(4), 261-261 (1975)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门