推荐产品
等級
for GC derivatization
品質等級
化驗
≥99.0% (GC)
≥99.0%
形狀
crystals
品質
LiChropur™
反應適用性
reagent type: derivatization reagent
reaction type: Acylations
技術
gas chromatography (GC): suitable
bp
135 °C/18 mmHg (lit.)
mp
48-51 °C (lit.)
49-50 °C
儲存溫度
2-8°C
SMILES 字串
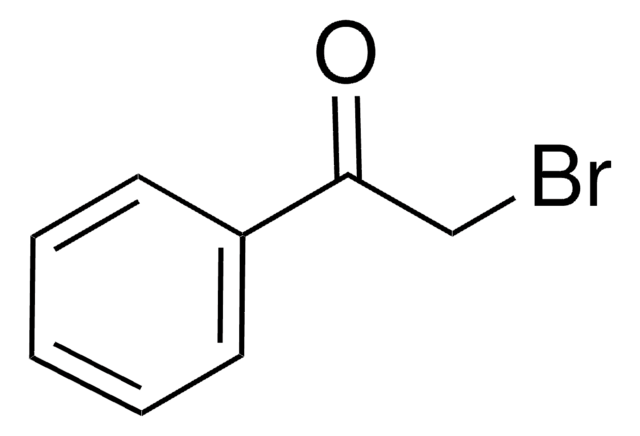
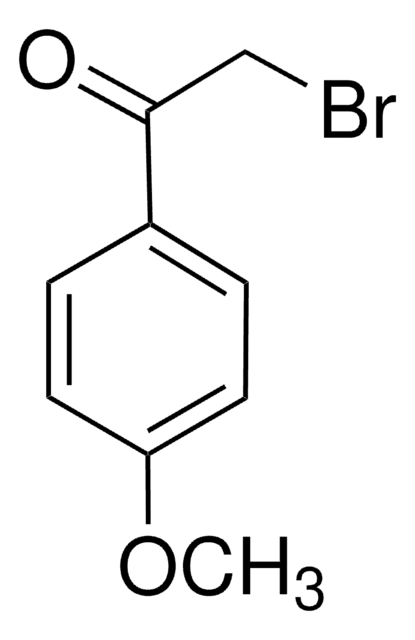
BrCC(=O)c1ccccc1
InChI
1S/C8H7BrO/c9-6-8(10)7-4-2-1-3-5-7/h1-5H,6H2
InChI 密鑰
LIGACIXOYTUXAW-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
2-溴苯乙酮是生物样品中脂肪酸检测常用的衍生化剂。
應用
与酸反应生成酯晶体
法律資訊
LiChropur is a trademark of Merck KGaA, Darmstadt, Germany
訊號詞
Danger
危險聲明
危險分類
Skin Corr. 1B
儲存類別代碼
6.1A - Combustible, acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
235.4 °F - closed cup
閃點(°C)
113 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
其他客户在看
High-performance liquid chromatography of fatty acids in biological samples.
Lima ES and Abdalla DSP
Analytica Chimica Acta, 465(1-2), 81-91 (2002)
P A Wender et al.
Organic letters, 1(13), 2117-2120 (2000-06-03)
[formula: see text] 4'-Bromoacetophenone derivatives which upon excitation can generate monophenyl radicals capable of hydrogen atom abstraction were investigated as photoinducible DNA cleaving agents. Pyrrolecarboxamide-conjugated 4'-bromoacetophenones were synthesized, and their DNA cleaving activities and sequence selectivities were determined.
Gulnur Arabaci et al.
Bioorganic & medicinal chemistry letters, 12(21), 3047-3050 (2002-10-10)
A series of alpha-haloacetophenone derivatives was tested for inhibition of protein tyrosine phosphatases SHP-1 and PTP1B. The results show that the bromides are much more potent than the corresponding chlorides, whereas the phenyl ring is remarkably tolerant to modifications. Derivatization
T Endoh et al.
Carcinogenesis, 17(3), 467-475 (1996-03-01)
Effects of inhibitors of arachidonic acid (AA) metabolism on the development of fatty liver, cirrhosis, glutathione-S-transferase placental form (GST-P)-positive nodules and the generation of 8-hydroxydeoxyguanosine (8-OHdG) and thiobarbituric acid-reactive substances (TBARS), caused by a choline-deficient, L-amino acid-defined (CDAA) diet, were
Mostafa A Hussein et al.
Acta pharmaceutica (Zagreb, Croatia), 59(4), 365-382 (2009-11-19)
5-Acyl-8-hydroxyquinoline-2-(3'-substituted-4'-aryl-2,3-dihydrothiazol-2'-ylidene)hydrazones, 5a-e to 10a-c, were prepared by the reaction of appropriate 5-acyl-8-hydroxyquinoline-4-substituted thiosemicarbazones 3a-e and phenacyl bromides 4a-e. Structures of the new compounds were verified on the basis of spectral and elemental analyses. Twenty-eight new compounds were tested for their
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门