推荐产品
等級
analytical standard
品質等級
產品線
PESTANAL®
化驗
98-100% (GC)
儲存期限
limited shelf life, expiry date on the label
技術
HPLC: suitable
gas chromatography (GC): suitable
bp
204-210 °C (lit.)
mp
54-58 °C (lit.)
應用
agriculture
environmental
格式
neat
SMILES 字串
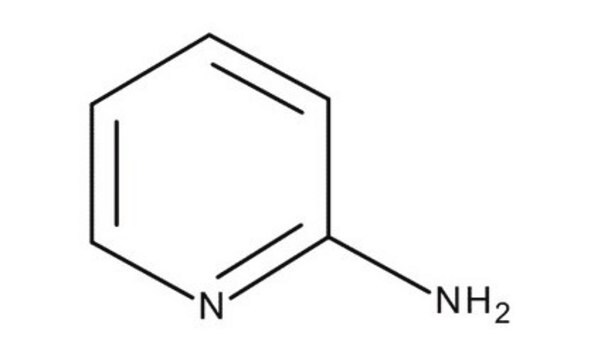
Nc1ccccn1
InChI
1S/C5H6N2/c6-5-3-1-2-4-7-5/h1-4H,(H2,6,7)
InChI 密鑰
ICSNLGPSRYBMBD-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
相关类别
一般說明
2-Aminopyridine can be called as structural motifs, which can be encountered in a variety of useful compounds, that includes fluorescent organic materials and therapeutics. It can undergo condensation with an aromatic aldehyde to give the corresponding schiff′s base.
應用
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
推薦產品
Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
法律資訊
PESTANAL is a registered trademark of Merck KGaA, Darmstadt, Germany
訊號詞
Danger
危險分類
Acute Tox. 3 Dermal - Acute Tox. 3 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1A
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
197.6 °F - closed cup
閃點(°C)
92 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Pd?PEPPSI?IPentCl: A Useful Catalyst for the Coupling of 2?Aminopyridine Derivatives
Khadra A, et al.
Chemistry?A European Journal, 23, 3206-3212 (2017)
A novel approach towards design, synthesis and evaluation of some Schiff base analogues of 2-aminopyridine and 2-aminobezothiazole against hepatocellular carcinoma
Chacko S and Samanta S
Biomedicine and Pharmacotherapy, 89, 162-176 (2017)
Honggen Wang et al.
Journal of the American Chemical Society, 132(38), 13217-13219 (2010-09-09)
A novel and efficient synthesis of pyrido[1,2-a]benzimidazoles through direct intramolecular aromatic C-H amination of N-aryl-2-aminopyridines has been developed. The reaction, cocatalyzed by Cu(OAc)(2) and Fe(NO(3))(3)·9H(2)O, is carried out in DMF under a dioxygen atmosphere. Diversified pyrido[1,2-a]benzimidazoles containing various substitution patterns
Thomas Zengeya et al.
Angewandte Chemie (International ed. in English), 51(50), 12593-12596 (2012-11-06)
Peptide nucleic acids containing thymidine and 2-aminopyridine (M) nucleobases form stable and sequence-selective triple helices with double-stranded RNA at physiologically relevant conditions. The M-modified PNA showed unique RNA selectivity by having two orders of magnitude higher affinity for the double-stranded
Philipp Ottiger et al.
The Journal of chemical physics, 136(17), 174308-174308 (2012-05-16)
The S(1)/S(2) state exciton splittings of symmetric doubly hydrogen-bonded gas-phase dimers provide spectroscopic benchmarks for the excited-state electronic couplings between UV chromophores. These have important implications for electronic energy transfer in multichromophoric systems ranging from photosynthetic light-harvesting antennae to photosynthetic
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门