推荐产品
等級
analytical standard
品質等級
產品線
PESTANAL®
儲存期限
limited shelf life, expiry date on the label
技術
HPLC: suitable
gas chromatography (GC): suitable
應用
agriculture
environmental
形式
neat
SMILES 字串
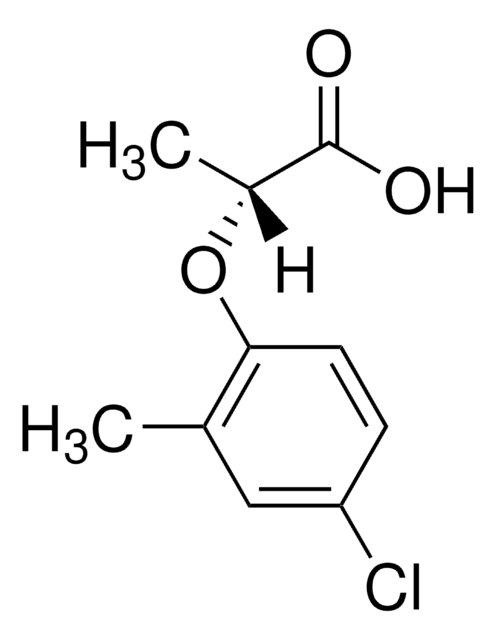
CC(Oc1ccc(Cl)cc1C)C(O)=O
InChI
1S/C10H11ClO3/c1-6-5-8(11)3-4-9(6)14-7(2)10(12)13/h3-5,7H,1-2H3,(H,12,13)
InChI 密鑰
WNTGYJSOUMFZEP-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
法律資訊
PESTANAL is a registered trademark of Merck KGaA, Darmstadt, Germany
未找到合适的产品?
试试我们的产品选型工具.
訊號詞
Danger
危險分類
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Skin Irrit. 2
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
其他客户在看
Pablo J Maid et al.
Journal of clinical rheumatology : practical reports on rheumatic & musculoskeletal diseases, 24(4), 177-182 (2017-12-13)
Biologic agents may induce immune responses that could impact drug action. The aims of this study were to assess antidrug antibodies (ADAs) in patients with rheumatoid arthritis (RA) from Argentina treated with etanercept, adalimumab, or infliximab at a single visit
Kohei Shitara et al.
Oncotarget, 8(45), 79546-79555 (2017-11-08)
SAR125844 is a potent and selective inhibitor of the c-Met kinase receptor. This was an open-label, phase I, multicenter, dose-escalation, and dose-expansion trial of SAR125844 in Asian patients with solid tumors, a subgroup of whom had gastric cancer and
Julien Taieb et al.
Drugs, 79(13), 1375-1394 (2019-07-28)
The approval of targeted therapies for metastatic colorectal cancer (mCRC) has led to important improvements in patient outcomes. However, it is still necessary to increase individualisation of treatments based on tumour genetic profiles to optimise efficacy, while minimising toxicity. As
Régis Peffault de Latour et al.
British journal of haematology, 191(3), 476-485 (2020-05-26)
Ravulizumab, a novel long-acting complement component 5 (C5) inhibitor administered every 8 weeks (q8w), was non-inferior to eculizumab for all efficacy outcomes in two randomised, open-label, phase 3 trials in C5 inhibitor-naïve (Study 301) and eculizumab-experienced (Study 302) adult patients
Yutaka Osuga et al.
Obstetrics and gynecology, 133(3), 423-433 (2019-02-12)
To investigate the noninferiority of relugolix compared with leuprorelin acetate in reducing heavy menstrual bleeding associated with uterine leiomyomas. In a double-blind, double-dummy trial, premenopausal women with uterine leiomyomas and heavy menstrual bleeding defined as a pictorial blood loss assessment
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门