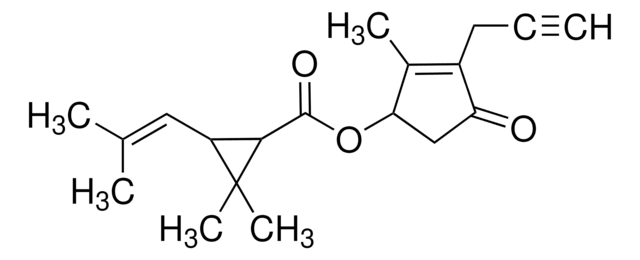
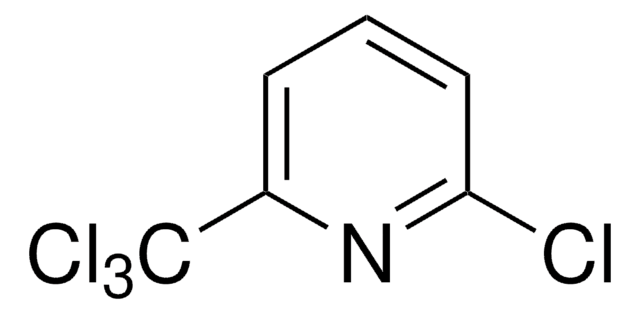
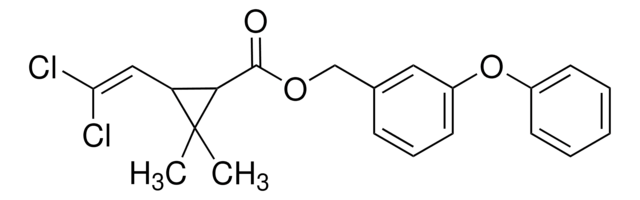
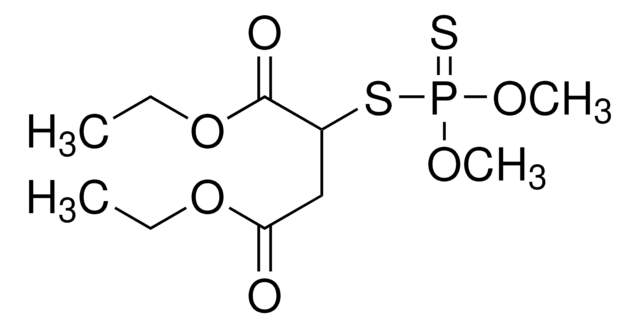
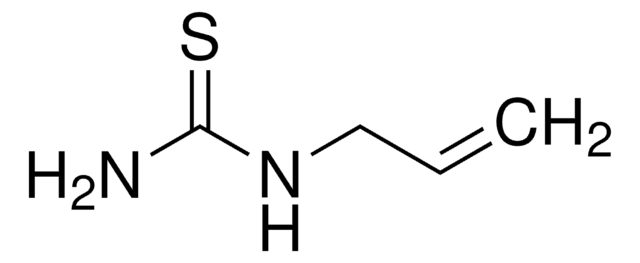
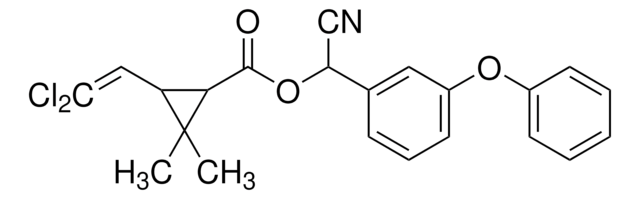
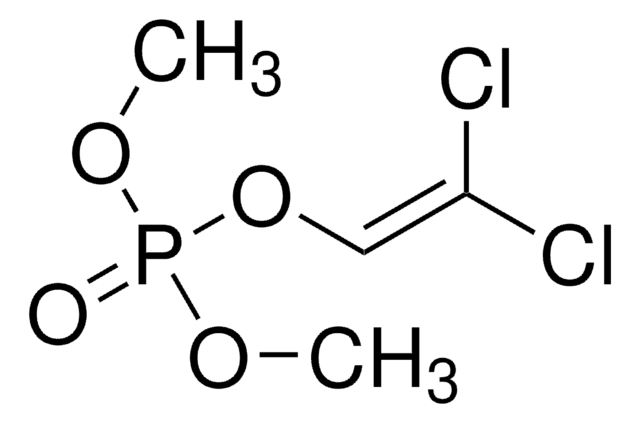
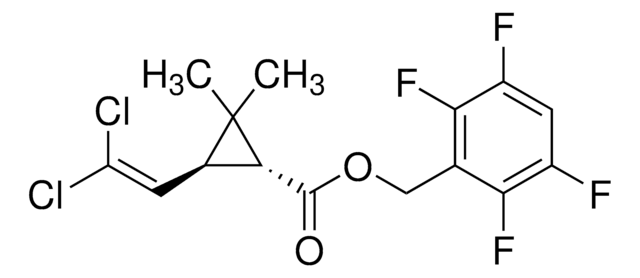
推荐产品
等級
analytical standard
品質等級
產品線
PESTANAL®
儲存期限
limited shelf life, expiry date on the label
技術
HPLC: suitable
NMR: suitable
gas chromatography (GC): suitable
mp
61-65 °C
適合性
passes test for identity (NMR)
應用
agriculture
environmental
格式
neat
SMILES 字串
Clc1cccc(n1)C(Cl)(Cl)Cl
InChI
1S/C6H3Cl4N/c7-5-3-1-2-4(11-5)6(8,9)10/h1-3H
InChI 密鑰
DCUJJWWUNKIJPH-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
相关类别
一般說明
Nitrapyrin is a nitrification inhibitor, which can also undergo hydrolysis to form 6-chloropicolinic acid, that can inhibit methane oxidation by M. trichosporium OB3b.
應用
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
推薦產品
Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
法律資訊
PESTANAL is a registered trademark of Merck KGaA, Darmstadt, Germany
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Aquatic Chronic 2
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 2
閃點(°F)
212.0 °F - closed cup
閃點(°C)
100 °C - closed cup
個人防護裝備
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
The effect of nitrapyrin and chloropicolinic acid on ammonium oxidation by Nitrosomonas europaea
Powell, S.J.; JProsser, J.I.;
FEMS Microbiology Letters, 28, 51-54 (1985)
S J Powell et al.
Journal of general microbiology, 137(8), 1923-1929 (1991-08-01)
Nitrate production by Nitrosomonas europaea in inorganic liquid medium containing ammonium was limited by reduction in pH. In the presence of montmorillonite and vermiculite, expanding clays with high cation-exchange-capacity (CEC), nitrite yield was increased, ammonia oxidation continued at pH values
Q R Shen et al.
Chemosphere, 50(6), 747-753 (2003-04-12)
Because low concentration of nitrite could be toxic to biological systems and high amounts of nitrite have been observed in a river of northern China since 1990, nitrite from agricultural soil sources should be investigated. In this paper, effects of
Distribution of nitrapyrin [12-chloro-6-(trichloromethyl)-pyridine] and 2-chloro-6-(dichloromethyl)-pyridine in red beet treated with nitrapyrin.
H Kallio et al.
Journal of the science of food and agriculture, 33(5), 451-455 (1982-05-01)
N M Berdasco et al.
Fundamental and applied toxicology : official journal of the Society of Toxicology, 11(3), 464-471 (1988-10-01)
Pregnant Fischer 344 rats and New Zealand White rabbits were orally administered 0, 5, 15, or 50 mg nitrapyrin/kg/day on Gestation Days 6 through 15 (rats) or 0, 3, 10, or 30 mg/kg/day on Gestation Days 6 through 18 (rabbits).
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门