推荐产品
等級
analytical standard
品質等級
產品線
VETRANAL®
技術
HPLC: suitable
gas chromatography (GC): suitable
應用
forensics and toxicology
veterinary
形式
neat
儲存溫度
2-8°C
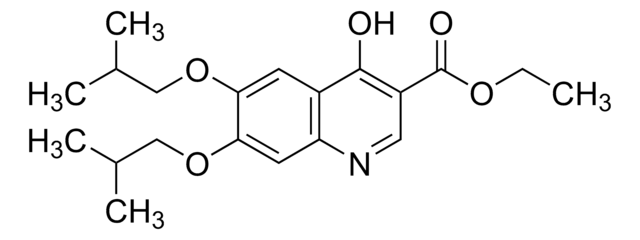
SMILES 字串
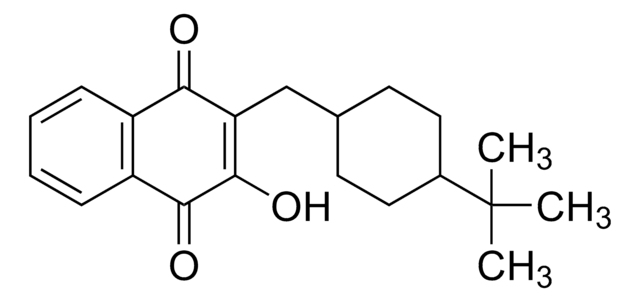
CC(C)(C)C1CCC(CC1)CC2=C(O)C(=O)c3ccccc3C2=O
InChI
1S/C21H26O3/c1-21(2,3)14-10-8-13(9-11-14)12-17-18(22)15-6-4-5-7-16(15)19(23)20(17)24/h4-7,13-14,24H,8-12H2,1-3H3
InChI 密鑰
KLLIVCPQDTYMLC-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
相关类别
應用
有关合适的仪器技术的更多信息,请参阅产品的分析证书。想要获得更多支持,请联系技术服务部。
法律資訊
VETRANAL is a registered trademark of Merck KGaA, Darmstadt, Germany
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Gantala Venkatesh et al.
Drug development and industrial pharmacy, 36(6), 735-745 (2010-02-09)
The aim of this study was to prepare a lipid-based self-microemulsifying drug delivery system (SMEDDS) to increase the solubility and oral bioavailability of a poorly water-soluble compound, buparvaquone (BPQ). The solubility of BPQ was determined in various vehicles, and pseudo-ternary
Juliana Q Reimão et al.
Experimental parasitology, 130(3), 195-199 (2012-01-28)
The objective of this study was to develop a novel liposomal formulation, containing phosphatidylserine (PS), of buparvaquone (BPQ) and to evaluate its in vivo effectiveness in Leishmania (L.) infantum chagasi-infected hamsters. The activity of BPQ was evaluated against both the
G R Muraguri et al.
Research in veterinary science, 81(1), 119-126 (2005-11-18)
East Coast fever, caused by the protozoan parasite Theileria parva, kills about 600,000 cattle annually in Africa. The hydroxynaphthoquinone compound buparvaquone (BPQ) is curative. Sixteen calves were infected with T. parva. On manifestation of disease symptoms, eight were injected with
Bariş Saruhan et al.
Turkiye parazitolojii dergisi, 32(4), 317-321 (2009-01-22)
The aim of this study was to examine the efficacy of buparvaquone (Buparvon, ALKE, Istanbul) in the treatment of theileriosis in cattle. The causative agent T. annulata causes direct and indirect gross economical loss in Turkey. Theileriosis was microscopically diagnosed
Tracy Garnier et al.
The Journal of pharmacy and pharmacology, 59(1), 41-49 (2007-01-18)
As the part of a study to develop buparvaquone (BPQ) formulations for the treatment of cutaneous leishmaniasis, the topical delivery of BPQ and one of its prodrugs from a range of formulations was evaluated. In previous studies, BPQ and its
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门