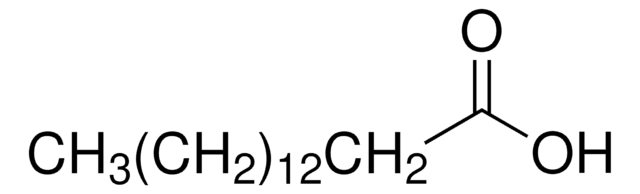推荐产品
材料
EPDM rubber seal
PPO/PS blend (core)
PPO/PS blend housing
TPE seal
composite regenerated cellulose (CRC) membrane
epoxy adhesive
polyester screen
polypropylene screen
polyurethane adhesive
品質等級
無菌
sterile; γ-irradiated
產品線
Pellicon®
特點
holdup volume 456 mL (in feed channel)
holdup volume 741 mL (in permeate channel)
包裝
package of 1
參數
max. inlet pressure at 75 psi (5.1 bar) and 4-30 °C
max. transmembrane pressure at 45 psi (3.1 bar) and 4-30 °C
4-6 L/min-m2 flow rate
技術
ultrafiltration: suitable
直徑
10.3 cm (4.1 in.)
過濾面積
1.5 m2
螢幕尺寸
, Type C screen (coarse screen)
尺寸
43.7 cm (17.2 in.) , Length
雜質
≤5 ppm Meets TOC (in retentate effluent, after a WFI flush of 20 L/m²)
基質
Ultracel®
孔徑
300 kDa
pH值範圍
2-13
儲存溫度
15-25°C
正在寻找类似产品? 访问 产品对比指南
一般說明
The Pellicon® Capsule is the first of its kind -- a true single-use TFF device that comes ready to process in minutes. Self-contained, it employs an easy-to-use, holderless format and is supplied sterilized by irradiation.
Feed: AseptiQuik® G Connector
Permeate: AseptiQuik® G Connector
Retentate: AseptiQuik® G Connector
Permeate: AseptiQuik® G Connector
Permeate: AseptiQuik® G Connector
應用
Viral Gene Therapies, Vaccines, Concentration, Diafiltration, Buffer exchange, Protein purification, mRNA
特點和優勢
- Plug ′n play, holderless design - easy to install, safe to remove
- Gamma Sterilized and preseravtive-free - ready to process in minutes
- True single-use, self-contained capusle - fast, safe, and flexible batch turnaround
- Proven Ultracel® membrane and C screen - high recovery, superior mass transfer, solvent resistance
- Pellicon® TFF proven performance - true linear scalability within Pellicon® TFF families
包裝
A foam clamshell is inserted over the end caps on both ends of the capsule which is then double-bagged and vacuum sealed. The double-bagged capsule is placed on a tray and then individually boxed.
其他說明
The Emprove® Program complements the product portfolio through
- Comprehensive documentation to support qualification, risk assessment and process optimization needs.
- Consolidation of product specific testing, quality and regulatory information to simplify compliance requirements.
- Convenient 24/7 access to up-to-date product information.
法律資訊
AseptiQuik is a registered trademark of Colder Products Company
Emprove is a registered trademark of Merck KGaA, Darmstadt, Germany
Pellicon is a registered trademark of Merck KGaA, Darmstadt, Germany
ULTRACEL is a registered trademark of Merck KGaA, Darmstadt, Germany
相關產品
产品编号
说明
价格
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门