推荐产品
材料
316 stainless steel cannula (ASTM®)
PETG bottle
PureFlex™ polyethylene bottle
TPE tubing (Thermoplastic elastomer)
polyester body
silicone septum (platinum-cured)
品質等級
agency
according to ISO 11137 (sterilization)
according to ISO 146441
meets USP 88 biological reactivity (all component materials)
無菌
sterile; γ-irradiated
產品線
NovaSeptum® GO
特點
autoclavable: no
參數
-80-50 °C temp. range (-112-122 °F)
0.50 bar max. pressure (7.25 psi)
雜質
<2.15 EU/device bacterial endotoxins (LAL test)
接頭
outlet (Cap)
應用
bioburden testing
cell therapy
gene therapy
life science and biopharma
mAb
ophthalmics
parenterals
pathogen testing
pharma/biopharma processes
sterile sampling
viral therapy
儲存溫度
room temp
正在寻找类似产品? 访问 产品对比指南
一般說明
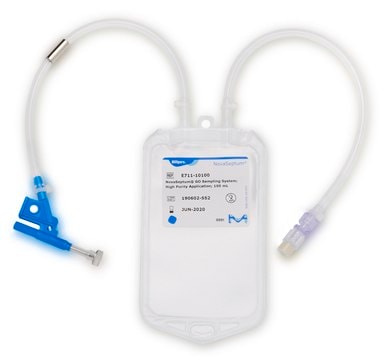
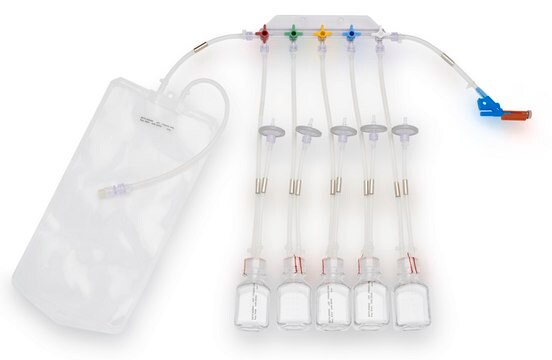
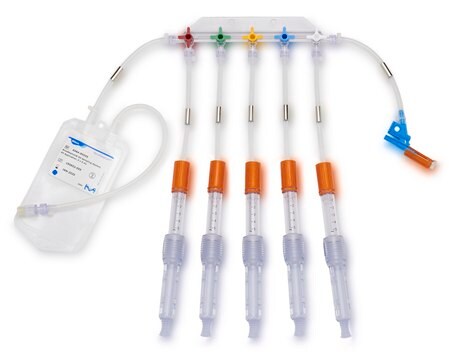
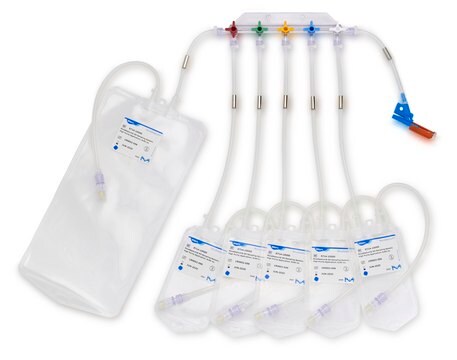
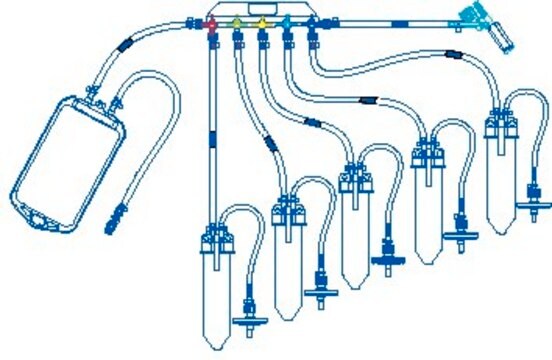
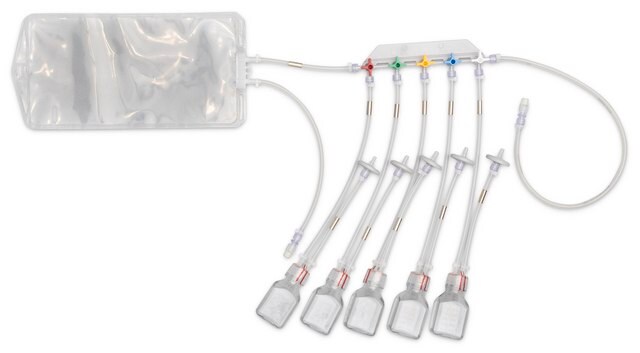
Device configuration: single Unit Sampling
The NovaSeptum® GO bottle sampling unit is intended for applications where high purity, such as low endotoxin levels, is required. This sampling unit is available with a 2 mm needle and must be used with a NovaSeptum® GO holder. The bio-neutral plastic bottles are ideal for all tests and available in a range of sizes. A closed, sterile sampling method can significantly reduce risk of cross contamination. Ideal for sampling from aseptic and sterile processes, the NovaSeptum® GO sterile sampling system provides consistently representative samples, safely and securely.
應用
Endotoxin
Bioburden -
Archiving
Bioburden -
Archiving
包裝
E871-80060 (60 ml bottle, 40 units per box)
E871-80125 (125 ml bottle, 25 units per box)
E871-80250 (250 ml bottle, 20 units per box)
E871-80500 (500 ml bottle, 12 units per box)
E871-80125 (125 ml bottle, 25 units per box)
E871-80250 (250 ml bottle, 20 units per box)
E871-80500 (500 ml bottle, 12 units per box)
成分
- Septum: Platinum-cured silicone
- Body: Polyester
- Cannula: ASTM® 316 L Stainless steel
- Fluid contact layer (bottle): Polyethylene Terephtalate Glycol(PETG)
- Tubing: Thermoplastic elastomer (TPE)
- Outlet Tubing: Cap
準備報告
Units are integrity tested at regular intervals during manufacturing.
Aqueous extraction contains < 2.15 EU per device as determined using the Limulus Amebocyte Lysate (LAL) test.
Aqueous extraction contains < 2.15 EU per device as determined using the Limulus Amebocyte Lysate (LAL) test.
法律資訊
ASTM is a registered trademark of American Society for Testing and Materials
NOVASEPTUM is a registered trademark of Merck KGaA, Darmstadt, Germany
PUREFLEX is a trademark of Merck KGaA, Darmstadt, Germany
未找到合适的产品?
试试我们的产品选型工具.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门