推荐产品
产品名称
Camptothecin, Camptotheca acuminata, A cell-permeable DNA topoisomerase I inhibitor.
品質等級
描述
Merck USA index - 14, 1735
化驗
≥95% (HPLC)
形狀
solid
製造商/商標名
Calbiochem®
儲存條件
OK to freeze
顏色
pale yellow
溶解度
DMSO: 10 mg/mL
methanol: 40 mg/mL
運輸包裝
ambient
儲存溫度
2-8°C
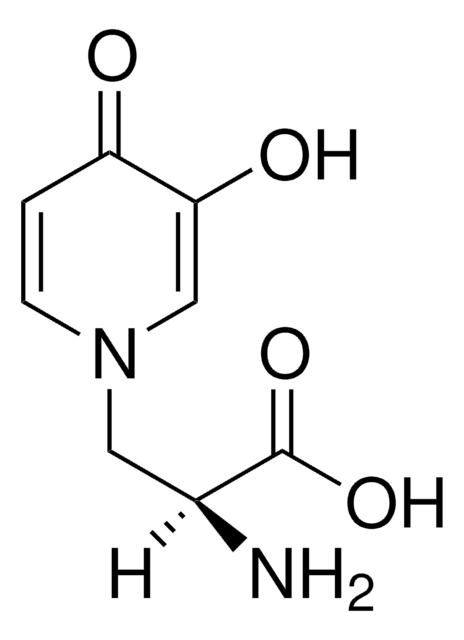
SMILES 字串
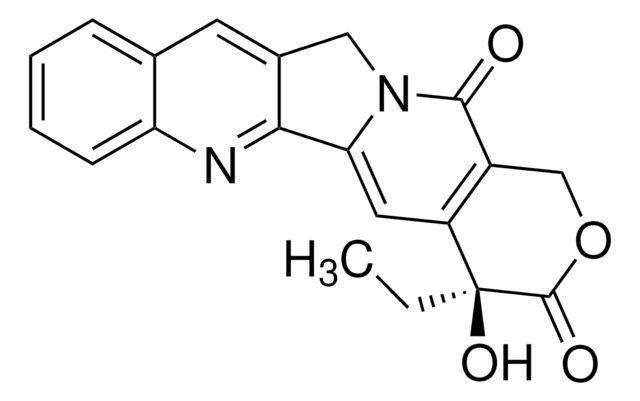
N21Cc3c(nc5c(c3)cccc5)C2=CC4=C(COC(=O)[C@]4(O)CC)C1=O
InChI
1S/C20H16N2O4/c1-2-20(25)14-8-16-17-12(7-11-5-3-4-6-15(11)21-17)9-22(16)18(23)13(14)10-26-19(20)24/h3-8,25H,2,9-10H2,1H3/t20-/m0/s1
InChI 密鑰
VSJKWCGYPAHWDS-FQEVSTJZSA-N
一般說明
A cell-permeable DNA topoisomerase I inhibitor. Exhibits anti-leukemic and antitumor properties. Induces apoptosis in HL-60 cells and mouse thymocytes. Arrests cells at the G2/M phase.
A cell-permeable, reversible DNA topoisomerase I inhibitor that binds to and stabilizes the topoisomerase-DNA covalent complex. Possesses antileukemic and antitumor properties. Inhibits Tat-mediated transactivation of HIV-1. Cytostatic for non-tumorigenic cells but cytotoxic for tumorigenic cells. Induces apoptosis of HL-60 cells and mouse thymocytes.
生化/生理作用
Cell permeable: yes
Primary Target
DNA topoisomerase 1
DNA topoisomerase 1
Product does not compete with ATP.
Reversible: no
警告
Toxicity: Toxic & Carcinogenic / Teratogenic (G)
準備報告
Stock solutions made with methanol will remain cloudy.
重構
Following reconstitution, aliquot and freeze (-20°C). Stock solutions are stable for up to 2 months at -20°C.
其他說明
Due to the nature of the Hazardous Materials in this shipment, additional shipping charges may be applied to your order. Certain sizes may be exempt from the additional hazardous materials shipping charges. Please contact your local sales office for more information regarding these charges.
Jones, C.B., et al. 1997. Cancer Chemother. Pharmacol.40, 475.
Staron, K., et al. 1994. Carcinogenesis 15, 2953.
Tanizawa, A., et al. 1994. J. Natl. Cancer Inst. 86, 836.
Gorczyca, W., et al. 1993. Toxicol. Lett.67, 249.
Onishi, Y., et al. 1993. Biochim. Biophys. Acta1175, 147.
Pantazis, P., et al. 1993. Int. J. Cancer53, 863.
Morham, S.G., and Shuman, S. 1992. J. Biol. Chem.267, 15984.
Hertzberg, R.P., et al. 1990. Biochem. J.28, 4629.
Hertzberg, R.P., et al. 1990. J. Biol. Chem.265, 19287.
Hsiang, Y.H., et al. 1985. J. Biol. Chem.260, 14873.
Staron, K., et al. 1994. Carcinogenesis 15, 2953.
Tanizawa, A., et al. 1994. J. Natl. Cancer Inst. 86, 836.
Gorczyca, W., et al. 1993. Toxicol. Lett.67, 249.
Onishi, Y., et al. 1993. Biochim. Biophys. Acta1175, 147.
Pantazis, P., et al. 1993. Int. J. Cancer53, 863.
Morham, S.G., and Shuman, S. 1992. J. Biol. Chem.267, 15984.
Hertzberg, R.P., et al. 1990. Biochem. J.28, 4629.
Hertzberg, R.P., et al. 1990. J. Biol. Chem.265, 19287.
Hsiang, Y.H., et al. 1985. J. Biol. Chem.260, 14873.
法律資訊
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral - Muta. 1B
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Frederick A Partridge et al.
Molecules (Basel, Switzerland), 26(20) (2021-10-24)
Mosquito-borne viruses including dengue, Zika, and Chikungunya viruses, and parasites such as malaria and Onchocerca volvulus endanger health and economic security around the globe, and emerging mosquito-borne pathogens have pandemic potential. However, the rapid spread of insecticide resistance threatens our
Wade R Gutierrez et al.
JCI insight (2022-10-14)
The DNA methyltransferase inhibitor decitabine has classically been used to reactivate silenced genes and as a pre-treatment for anti-cancer therapies. In a new variation of this idea, this study explores the concept of adding low-dose decitabine following administration of chemotherapy
Md Akram Hossain et al.
Frontiers in cell and developmental biology, 9, 738502-738502 (2021-11-20)
The maintenance of genome integrity and fidelity is vital for the proper function and survival of all organisms. Recent studies have revealed that APE2 is required to activate an ATR-Chk1 DNA damage response (DDR) pathway in response to oxidative stress
Elisabetta Rubini et al.
Molecules (Basel, Switzerland), 26(23) (2021-12-11)
The β-isomer of hexachlorocyclohexane (β-HCH) is a globally widespread pollutant that embodies all the physicochemical characteristics of organochlorine pesticides, constituting an environmental risk factor for a wide range of noncommunicable diseases. Previous in vitro studies from our group disclosed the
Jianming Wang et al.
Cell reports, 34(7), 108759-108759 (2021-02-18)
As transcription and replication use DNA as substrate, conflicts between transcription and replication can occur, leading to genome instability with direct consequences for human health. To determine how the two processes are coordinated throughout S phase, we characterize both processes
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门