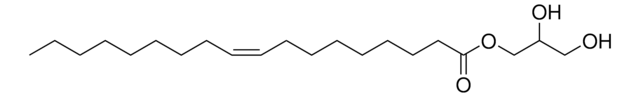
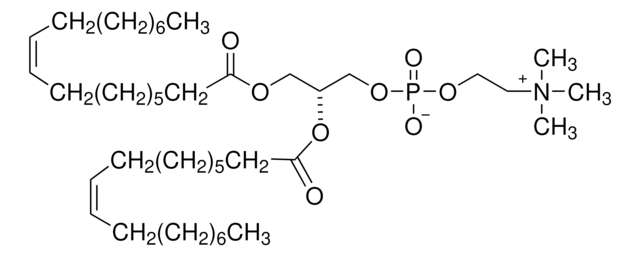
推荐产品
化驗
>99% (TLC)
形狀
liquid
包裝
pkg of 1 × 5 mL (800815C-10mg)
pkg of 1 × 5 mL (800815C-25mg)
pkg of 1 × 8 mL (800815C-200mg)
製造商/商標名
Avanti Research™ - A Croda Brand 800815C
濃度
2 mg/mL (800815C-10mg)
25 mg/mL (800815C-200mg)
5 mg/mL (800815C-25mg)
脂質類型
neutral glycerides
neutral lipids
運輸包裝
dry ice
儲存溫度
−20°C
一般說明
In biochemical signaling, diacylglycerol (DAG) functions as a second messenger signaling lipid, and is a product of the hydrolysis of the phospholipid PIP2 (phosphatidylinositolbisphosphate) by the enzyme phospholipase C (PLC) (a membrane-bound enzyme) that, through the same reaction, produces inositol trisphosphate (IP3). Although inositol trisphosphate (IP3) diffuses into the cytosol, DAG remains within the plasma membrane due to its hydrophobic properties. IP3 stimulates the release of calcium ions from the smooth endoplasmic reticulum, whereas DAG is a physiological activator of protein kinase C (PKC). The production of DAG in the membrane facilitates translocation of PKC from the cytosol to the plasma membrane.
Diacylglycerol mimicks the effects of the tumor-promoting compounds phorbol esters.
Diacylglycerol mimicks the effects of the tumor-promoting compounds phorbol esters.
應用
16:0-18:1 DG has been used to spike brain samples for mass spectrometric analysis. It may be used as benchmark dataset lipid in collision induced dissociation tandem mass spectrometry (CID-MS/MS) experiments and in in vitro diacylglycerol kinase assay.
包裝
30 mL Amber Narrow Mouth Glass Bottle with Screw Cap (800815C-10mg)
30 mL Amber Narrow Mouth Glass Bottle with Screw Cap (800815C-200mg)
30 mL Amber Narrow Mouth Glass Bottle with Screw Cap (800815C-25mg)
儲存和穩定性
Diacylglycerols are conveniently stored in chloroform solutions in glass vials with PTFE-lined caps at -20°C. Under these conditions acyl migration is minimal. Avoid plastic when handling chloroform solutions.
其他說明
Delivery to cells:
Dry samples of diacylglycerol in chloroform, using a stream of nitrogen. Dissolve the residue in an appropriate volume of ethanol or DMSO, then dilute to the desired aqueous medium.
Dry samples of diacylglycerol in chloroform, using a stream of nitrogen. Dissolve the residue in an appropriate volume of ethanol or DMSO, then dilute to the desired aqueous medium.
Effective concentration:
Most biological responses saturate at 20 to 250 μM sn-1,2-dioctanoylglycerol. Only sn-1,2 isomers appear to be active.
Most biological responses saturate at 20 to 250 μM sn-1,2-dioctanoylglycerol. Only sn-1,2 isomers appear to be active.
Precaution: Since short chain Diacylglycerols mimic effects of the tumor-promoting phorbol diesters in a number of biological systems, extra care should be employed in their handling. Treatment of solutions, vessels and other articles with 1N NaOH before washing or discarding will destroy diacylglycerols.
法律資訊
Avanti Research is a trademark of Avanti Polar Lipids, LLC
訊號詞
Danger
危險分類
Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Carc. 2 - Eye Irrit. 2 - Repr. 2 - Skin Irrit. 2 - STOT RE 1 - STOT SE 3
標靶器官
Central nervous system, Liver,Kidney
水污染物質分類(WGK)
WGK 3
CD1a-autoreactive T cells recognize natural skin oils that function as headless antigens
De Jong A, et al.
Nature Immunology, 15(2), 177-177 (2014)
Diacylglycerol kinase delta and sphingomyelin synthase-related protein functionally interact via their sterile alpha motif domains
Murakami C, et al.
The Journal of Biological Chemistry, 295(10), 2932-2947 (2020)
Quantification of signaling lipids by nano-electrospray ionization tandem mass spectrometry (Nano-ESI MS/MS)
Haag M, et al.
Metabolites, 2(1), 57-76 (2012)
Chiaki Murakami et al.
The Journal of biological chemistry, 295(10), 2932-2947 (2020-01-26)
The δ isozyme of diacylglycerol kinase (DGKδ) plays critical roles in lipid signaling by converting diacylglycerol (DG) to phosphatidic acid (PA). We previously demonstrated that DGKδ preferably phosphorylates palmitic acid (16:0)- and/or palmitoleic acid (16:1)-containing DG molecular species, but not
Xinxing Zhang et al.
Journal of the American Chemical Society (2018-11-22)
Nature carefully designs the components of amphiphile-composed monolayer and bilayer membranes to deliver specific functions. The compositions of these interfacial layered structures are so delicate that minute modifications can result in huge changes in function. Great efforts have been expended
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门