推荐产品
生物源
synthetic
品質等級
化驗
96%
折射率
n20/D 1.465 (lit.)
bp
88-91.5 °C (lit.)
密度
1.065 g/mL at 25 °C (lit.)
應用
flavors and fragrances
文件
see Safety & Documentation for available documents
食物過敏原
no known allergens
感官的
meaty; roasted
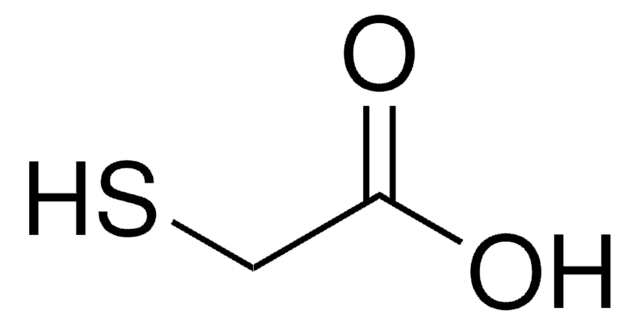
SMILES 字串
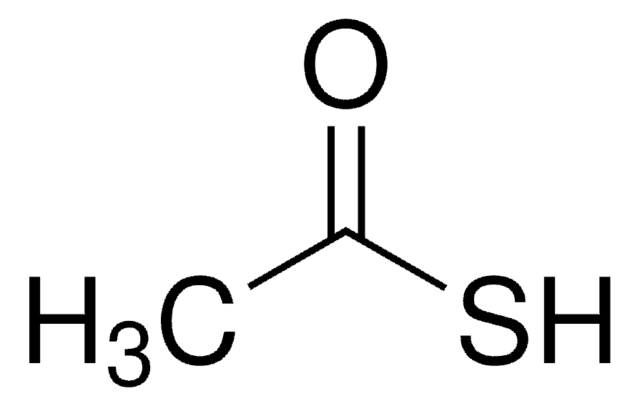
CC(S)=O
InChI
1S/C2H4OS/c1-2(3)4/h1H3,(H,3,4)
InChI 密鑰
DUYAAUVXQSMXQP-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
免責聲明
For R&D or non-EU Food use. Not for retail sale.
訊號詞
Danger
危險分類
Acute Tox. 3 Oral - Acute Tox. 4 Inhalation - Eye Dam. 1 - Flam. Liq. 2 - Skin Sens. 1
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
64.4 °F - closed cup
閃點(°C)
18 °C - closed cup
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Christina Wedemeyer-Exl et al.
Organic & biomolecular chemistry, 5(13), 2119-2128 (2007-06-22)
The thiol-dependent methylation of heptamethyl cob(II)yrinate 8r with methyl iodide and methyl tosylate was explored under a variety of conditions. The interaction of the heptamethyl cob(II)yrinate with a variety of thiols was monitored prior to the addition of the methylating
Sonia E Ulic et al.
The journal of physical chemistry. A, 112(27), 6211-6216 (2008-06-13)
Trifluorothioacetic acid-S-(trifluoromethyl)ester, CF3C(O)SCF3, was prepared by reacting CF3C(O)Cl and AgSCF3 at 50 degrees C. The compound was characterized by (13)C-, (19)F-NMR, UV, and vibrational spectroscopy as well as by gas electron diffraction (GED) and quantum chemical calculations (HF, MP2, and
I Vucenik et al.
Anticancer research, 21(2B), 1247-1255 (2001-06-09)
Based on a "field-effect" theory in colon carcinogenesis, and the expression of the disaccharide tumor marker D galactose-beta-[1-->3]-N-acetyl-D-galactosamine (Gal-GalNAc) in the rectal mucus of patients with cancer and precancer of the colon, Shamsuddin developed a simple, accurate, inexpensive, easy to
Ning Shangguan et al.
Journal of the American Chemical Society, 125(26), 7754-7755 (2003-06-26)
A new amide synthesis strategy based on a fundamental mechanistic revision of the reaction of thio acids and organic azides is presented. The data demonstrate that amines are not formed as intermediates in this reaction. Alternative mechanisms proceeding through a
Krista M Wager et al.
Organic letters, 13(15), 4052-4055 (2011-07-07)
A method for preparing benzyl aryl thioethers utilizing an in situ deprotection of benzyl thioacetates as an alternative to free thiols as starting materials has been developed and optimized. Good to excellent yields of diverse benzyl aryl thioethers are obtained
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门