About This Item
推荐产品
生物源
synthetic
等級
FG
Halal
agency
meets purity specifications of JECFA
法律遵循
EU Regulation 1334/2008 & 178/2002
FCC
FDA 21 CFR 163.110
FDA 21 CFR 163.111
FDA 21 CFR 163.112
FDA 21 CFR 184.1099
蒸汽密度
5.18 (vs air)
化驗
≥99.7%
形狀
crystalline powder
光學活性
[α]20/D +12.5°, c = 20 in H2O
自燃溫度
797 °F
mp
170-172 °C (lit.)
溶解度
water: soluble 150 g/L at 25 °C
正離子痕跡
As: ≤3 ppm
Cd: ≤1 ppm
Hg: ≤1 ppm
heavy metals (as Pb): ≤2 ppm
應用
flavors and fragrances
文件
see Safety & Documentation for available documents
食物過敏原
no known allergens
感官的
odorless
SMILES 字串
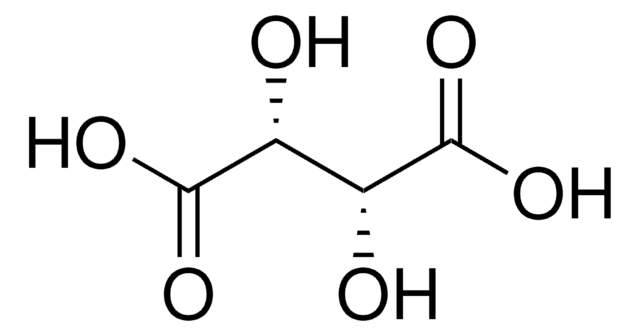
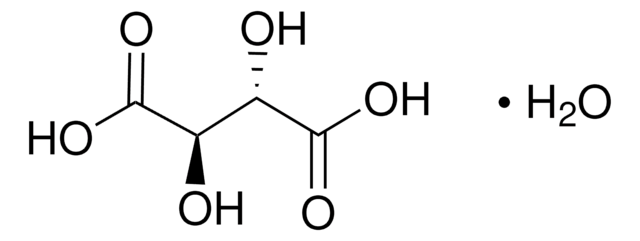
O[C@H]([C@@H](O)C(O)=O)C(O)=O
InChI
1S/C4H6O6/c5-1(3(7)8)2(6)4(9)10/h1-2,5-6H,(H,7,8)(H,9,10)/t1-,2-/m1/s1
InChI 密鑰
FEWJPZIEWOKRBE-JCYAYHJZSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
應用
- 太赫兹光谱法无损测定smartFilm和纸型片剂中L-酒石酸的结晶度。:本研究采用太赫兹光谱法评估创新制药应用中L-(+)-酒石酸的结晶度,改善了质量控制的无损检测方法(Ornik et al., 2020 May)。文章链接。
- 微粉化共晶干粉制剂改善难溶性伊曲康唑的肺部吸收。研究表明,在伊曲康唑的共晶制剂中使用L-(+)-酒石酸可改善其肺部吸收,这表明药物递送技术取得了重大进展(Karashima et al., 2017 Jun)。文章链接。
- 低溶性药物共无定形体的理化评价和可开发性评估以及与共晶体的比较。:本文讨论了L-(+)-酒石酸通过共无定形体系提高药物溶解度和生物利用度的作用,为药物制剂策略提供了重要见解(Yamamoto et al., 2016 Dec)。文章链接。
- 源自酒石酸的功能化聚碳酸酯:七元环状碳酸酯的酶促开环聚合。:本研究探讨了由L-(+)-酒石酸合成可生物降解聚合物,强调了其在开发环保材料方面的应用(Wu et al., 2008 Oct)。文章链接。
訊號詞
Danger
危險聲明
危險分類
Eye Dam. 1
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 1
閃點(°F)
302.0 °F - closed cup
閃點(°C)
150 °C - closed cup
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门