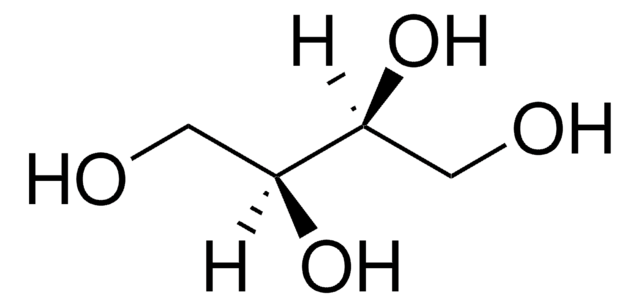
推荐产品
品質等級
化驗
99%
mp
69-71 °C (lit.)
SMILES 字串
c1ccc(cc1)\C=C(/c2ccccc2)c3ccccc3
InChI
1S/C20H16/c1-4-10-17(11-5-1)16-20(18-12-6-2-7-13-18)19-14-8-3-9-15-19/h1-16H
InChI 密鑰
MKYQPGPNVYRMHI-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
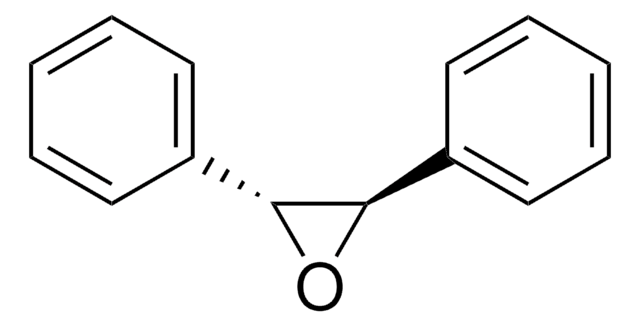
三苯乙烯是一种芳香烃,可作为起始材料,通过以氟手性锰络合物为催化剂的不对称环氧化反应制备2,2,3-三苯基环氧乙烷。也可在MnO2和NaOAc存在下,与乙酸酐反应制备二氢-4,5,5-三苯基-2(3H)-呋喃酮。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
S J Gatley et al.
International journal of radiation applications and instrumentation. Part B, Nuclear medicine and biology, 18(7), 769-775 (1991-01-01)
A triphenylethylene compound [1,1-bis(4-hydroxyphenyl)-2-iodo-2-phenylethylene; IBHPE] has been labeled by halodestannylation with 123I at a specific radioactivity of 13,200 Ci/mmol (by in vitro receptor assay) after HPLC purification. The corresponding 80mBr-labeled compound (BrBHPE), which has a 3-fold higher affinity for the
E Bignon et al.
FEBS letters, 271(1-2), 54-58 (1990-10-01)
The activation of type I (gamma), II (beta) and III (alpha) protein kinase C (PKC) subspecies by phosphatidylserine (PS) and diacylglycerol (DAG) is inhibited by micromolar concentrations of triphenylacrylonitrile (TPE) antiestrogens. TPE A (with p-hydroxy and p-diethylaminoethoxy groups on the
Synthesis of 1,1,2-triphenylethylenes and their antiproliferative effect on human cancer cell lines.
Lifang Zheng et al.
Anti-cancer drugs, 18(9), 1039-1044 (2007-08-21)
Tamoxifen analogs (1-3) and 1,1,2-triphenylethylenes (4-7) have been synthesized by the McMurry coupling reaction. Their antiproliferative effects on MCF-7 human breast-cancer cells, HO-8910 human ovarian-carcinoma cells, and (HL)-60 human promyelocytic-leukemia cells were studied by use of the colorimetric MTT assay
M Metzler et al.
American journal of clinical oncology, 14 Suppl 2, S30-S35 (1991-01-01)
The mechanisms of estrogen-induced cancer are still a matter of debate. Previous studies with stilbene estrogens and steroidal estrogens have shown that the in vitro transformation of primary Syrian hamster embryo (SHE) fibroblasts is a good experimental system for discriminating
C D van den Koedijk et al.
Biochemical pharmacology, 46(10), 1870-1872 (1993-11-17)
The binding affinity of derivatives of the triphenylethylene (TPE) antioestrogen tamoxifen and of steroidal compounds for human liver antioestrogen binding sites (AEBS) was compared with their binding affinity for rat liver AEBS. Despite the observation of some quantitative differences overall
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门