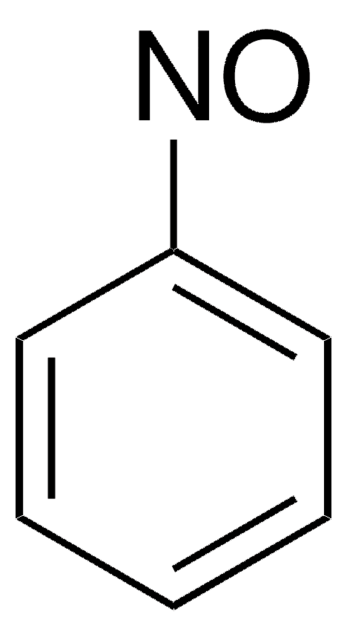
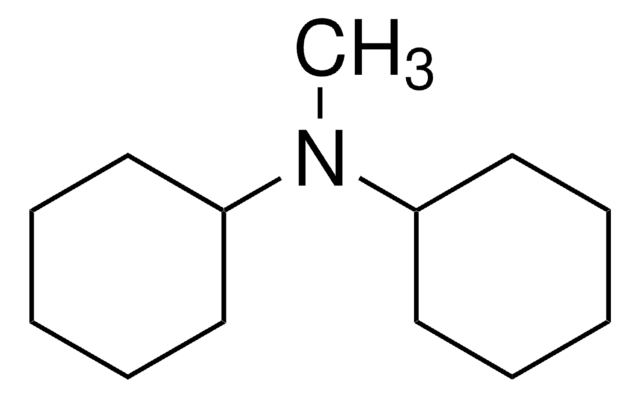
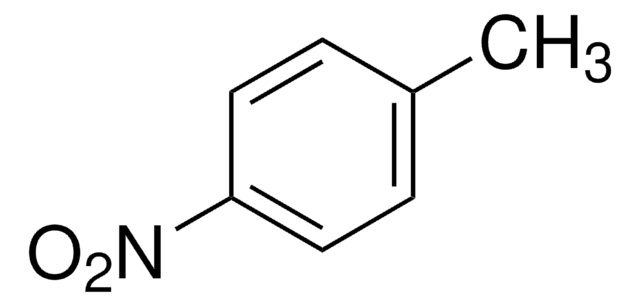
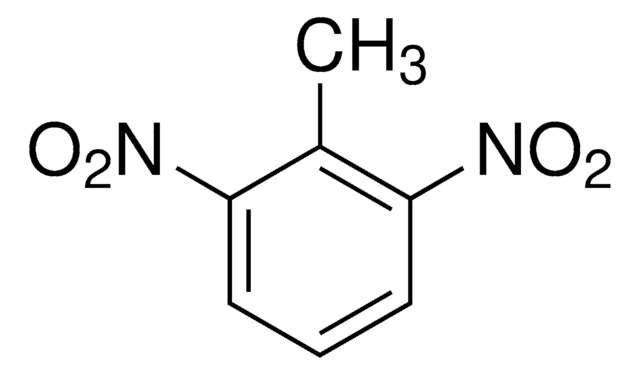
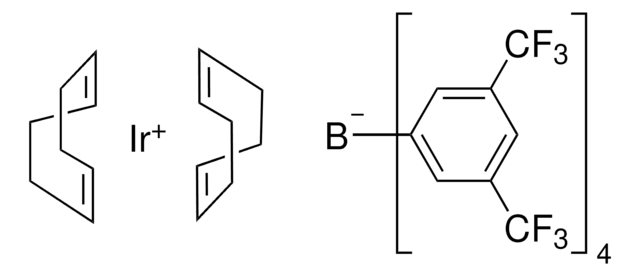
推荐产品
化驗
97%
形狀
solid
mp
72-75 °C (lit.)
儲存溫度
2-8°C
SMILES 字串
Cc1ccccc1N=O
InChI
1S/C7H7NO/c1-6-4-2-3-5-7(6)8-9/h2-5H,1H3
InChI 密鑰
TWLBRQVYXPMCFK-UHFFFAOYSA-N
訊號詞
Warning
危險分類
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
S S Hecht et al.
Cancer letters, 16(1), 103-108 (1982-05-01)
o-Toluidine hydrochloride and one of its metabolites, o-nitrosotoluene, were administered in the diet (0.028 mol/kg diet) to 2 groups of 30 male F-344 rats for 72 weeks. o-Nitrosotoluene induced significantly more tumors of the bladder (16/30 rats) and liver (20/30)
S S Hecht et al.
Cancer letters, 20(3), 349-354 (1983-10-01)
3,2'-Dimethyl-4-aminobiphenyl and 3,2'-dimethyl-4-nitrosobiphenyl were administered by subcutaneous injection in peanut oil to 2 groups of 15 male and 15 female Syrian golden hamsters. The total dose of each compound was 5.6 mmol/kg. In the group treated with 3,2'-dimethyl-4-aminobiphenyl, 24 animals
Y Ohkuma et al.
Archives of biochemistry and biophysics, 372(1), 97-106 (1999-11-24)
Mechanisms of DNA damage by metabolites of carcinogenic o-toluidine in the presence of metals were investigated by the DNA sequencing technique using (32)P-labeled human DNA fragments. 4-Amino-3-methylphenol, a major metabolite, caused DNA damage in the presence of Cu(II). Predominant cleavage
B Kulkarni et al.
Carcinogenesis, 4(10), 1275-1279 (1983-10-01)
High-performance liquid chromatography with electrochemical detection, a highly sensitive and specific method, was used to determine N-hydroxy-o-toluidine and o-toluidine in the urines of male F344 rats after the administration of 0.82 mmol/kg of o-toluidine or o-nitrosotoluene. In a six hour
Matthew A Cerny et al.
Archives of biochemistry and biophysics, 436(2), 265-275 (2005-03-31)
The inactivation of cytochrome P450 enzymes by cyclopropylamines has been attributed to a mechanism involving initial one-electron oxidation at nitrogen followed by scission of the cyclopropane ring leading to covalent modification of the enzyme. Herein, we report that in liver
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门