推荐产品
產品線
ReagentPlus®
化驗
99%
形狀
liquid
折射率
n20/D 1.592 (lit.)
bp
220-221 °C (lit.)
密度
1.063 g/mL at 25 °C (lit.)
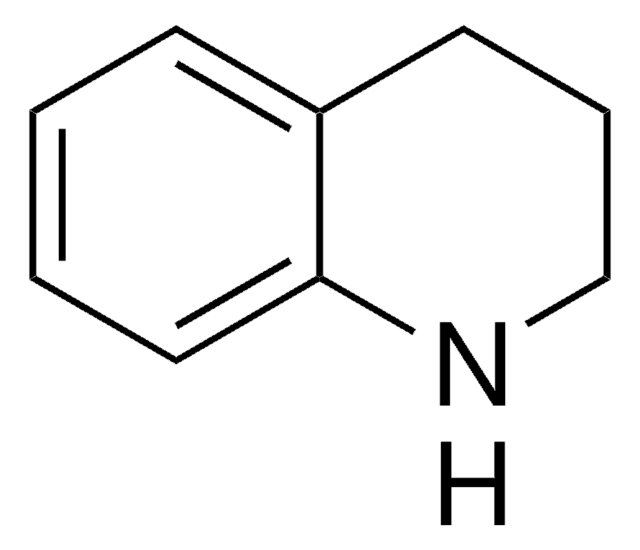
SMILES 字串
C1Cc2ccccc2N1
InChI
1S/C8H9N/c1-2-4-8-7(3-1)5-6-9-8/h1-4,9H,5-6H2
InChI 密鑰
LPAGFVYQRIESJQ-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
用作制备以下产品的反应物:
- NOD1 诱导的核因子-κB 活化抑制剂
- 1-磷酸鞘氨醇 4 (S1P4) 受体拮抗剂
- 细胞毒性细胞周期抑制剂
- 2-氨基吡啶类
- 用于蛋白激酶 C (PKC) 成像的 PET 试剂
- 治疗糖尿病高血糖症的钠依赖性葡萄糖协同转运蛋白 2 (SGLT2) 抑制剂
- α4β2-烟碱乙酰胆碱受体选择性部分激动剂
- mGlu4 阳性变构调节剂
- 细菌生物膜抑制剂
- 血清素 5-HT6 受体拮抗剂
法律資訊
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
199.4 °F - closed cup
閃點(°C)
93 °C - closed cup
個人防護裝備
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
其他客户在看
Matej Baláž et al.
Molecules (Basel, Switzerland), 24(18) (2019-09-22)
Performing solution-phase oximation reactions with hydroxylamine hydrochloride (NH2OH·HCl) carries significant risk, especially in aqueous solutions. In the present study, four N-substituted indole-3-carboxaldehyde oximes were prepared from the corresponding aldehydes by solvent-free reaction with NH2OH·HCl and a base (NaOH or Na2CO3)
Toshiharu Noji et al.
Organic letters, 15(8), 1946-1949 (2013-04-04)
A benzyne-mediated synthesis of substituted indolines and carbazoles was developed. The reaction includes generation of benzyne using Mg(TMP)2·2LiCl as a base, cyclization, and trapping the resulting organomagnesium intermediate with an electrophile to provide a series of substituted indolines and carbazoles
Gang He et al.
Organic letters, 14(12), 2944-2947 (2012-06-08)
An efficient method has been developed for the synthesis of indoline compounds from picolinamide (PA)-protected β-arylethylamine substrates via palladium-catalyzed intramolecular amination of ortho-C(sp(2))-H bonds. These reactions feature high efficiency, low catalyst loadings, mild operating conditions, and the use of inexpensive
Oktay Talaz et al.
Bioorganic & medicinal chemistry, 21(6), 1477-1482 (2012-11-06)
Several 1,4-bis(indolin-1-ylmethyl)benzene-based compounds containing substituents such as five, six and seven cyclic derivatives on indeno part (9a-c) were prepared and tested against two members of the pH regulatory enzyme family, carbonic anhydrase (CA). The inhibitory potencies of the compounds at
François Brucelle et al.
Organic letters, 14(12), 3048-3051 (2012-06-01)
A simple approach to prepare indolines and benzopyrrolizidinones from ortho-azidoallylbenzenes via a tandem radical addition/cyclization is described. The use of triethylborane to initiate and sustain the process provides the best results. Indolines are easily converted into the corresponding indoles by
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门