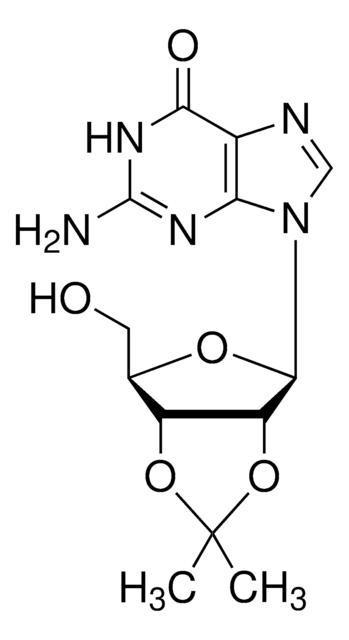
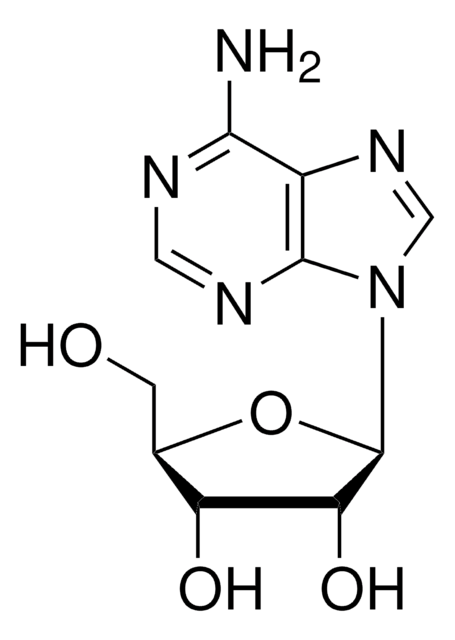
推荐产品
化驗
98%
光學活性
[α]20/D −98.5°, c = 1 in dioxane
mp
221-222 °C (lit.)
SMILES 字串
CC1(C)O[C@@H]2[C@@H](CO)O[C@H]([C@@H]2O1)n3cnc4c(N)ncnc34
InChI
1S/C13H17N5O4/c1-13(2)21-8-6(3-19)20-12(9(8)22-13)18-5-17-7-10(14)15-4-16-11(7)18/h4-6,8-9,12,19H,3H2,1-2H3,(H2,14,15,16)/t6-,8-,9-,12-/m1/s1
InChI 密鑰
LCCLUOXEZAHUNS-WOUKDFQISA-N
正在寻找类似产品? 访问 产品对比指南
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
M Brignoni et al.
Journal of cell science, 108 ( Pt 5), 1931-1943 (1995-05-01)
Madin-Darby canine kidney and other epithelial cell lines (e.g. Caco-2, MCF-10A and MCF-7) develop intracellular vacuoles composed of apical membrane displaying microvilli (VACs) when impaired from forming normal cell-to-cell contacts. In a previous publication, we showed that VACs are rapidly
I D Golovatskiĭ et al.
Ukrainskii biokhimicheskii zhurnal (1978), 58(3), 37-40 (1986-05-01)
Transformation of synthesized 2',3'-O-isopropylidene adenosine was studied in comparison with adenosine in rat liver homogenates. It is stated that 2',3'-O-isopropylidene adenosine is subjected to deamination similar to adenosine but less intensively. Due to deamination 2',3'-O-isopropylidene inosine is formed from 2',3'-O-isopropylidene
Pierangela Ciuffreda et al.
Nucleosides, nucleotides & nucleic acids, 26(10-12), 1311-1313 (2007-12-11)
2 ',3 '-Isopropylidene group can be used as a molecular scaffold for the introduction of modifications at 5 ' and 1 ' positions of adenosine and these modified nucleosides are used to evaluate the biocatalytic activity of adenosine and adenylate
Lindsay R Comstock et al.
The Journal of organic chemistry, 69(4), 1425-1428 (2004-02-14)
8-Azido-5'-aziridino-5'-deoxyadenosine (6), a novel cofactor mimic, was synthesized in nine steps from commercially available 2',3'-isopropylideneadenosine in approximately 4% overall yield. Crucial to this success was a very unorthodox phthalimide cleavage procedure, C8 azidation prior to aziridination and late stage alkylation
I D Golovatskiĭ et al.
Ukrainskii biokhimicheskii zhurnal (1978), 61(2), 64-69 (1989-03-01)
Transformation and uptake of [8-14C]-adenosine and its synthetic analog 2',3'-O-isopropylideneadenosine was studied in Zajdel hepatoma cells and their homogenates. Uptake and deamination of adenosine and 2',3'-O-isopropylideneadenosine by Zajdel hepatoma cells proceed differently. A small part of adenosine is phosphorylated and
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门