935417
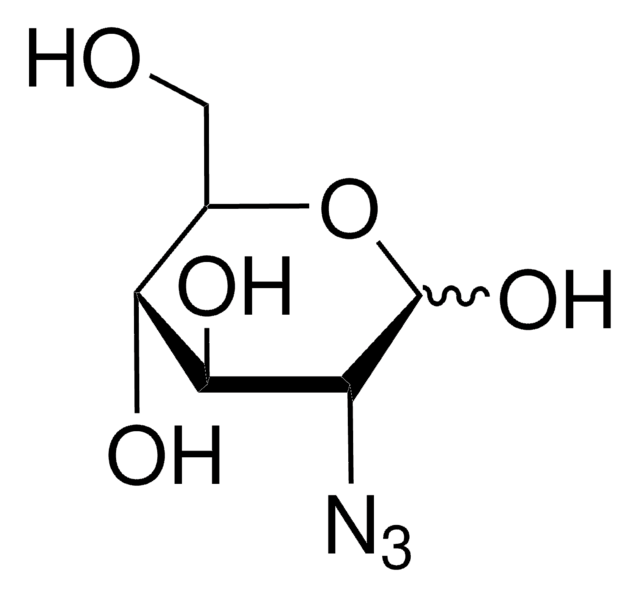
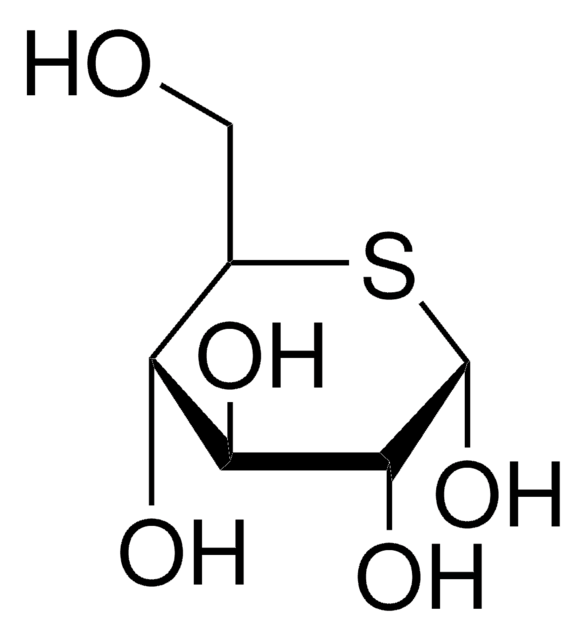
β-D-Glucopyranosyl azide
≥95%
别名:
2-Acetamido-2-deoxy-3,4,6-tri-O-acetyl-β-D-glucopyranosyl azide, 2-Acetamido-3,4,6-tri-O-acetyl-2-deoxy-β-D-glucopyranosyl azide, Glucopyranosyl azide, 2-acetamido-2-deoxy-, 3,4,6-triacetate, β-D-, Glucopyranosyl azide, 2-acetamido-2-deoxy-, triacetate
登录查看公司和协议定价
所有图片(1)
About This Item
推荐产品
品質等級
化驗
≥95%
形狀
powder or crystals
顏色
white to light yellow
bp
501.81 °C
mp
128-130 °C
密度
1.30 g/mL
儲存溫度
−20°C
SMILES 字串
[N-]=[N+]=NC1OC(COC(=O)C)C(OC(=O)C)C(OC(=O)C)C1NC(=O)C
InChI
InChI=1S/C14H20N4O8/c1-6(19)16-11-13(25-9(4)22)12(24-8(3)21)10(5-23-7(2)20)26-14(11)17-18-15/h10-14H,5H2,1-4H3,(H,16,19)/t10-,11-,12-,13-,14-/m1/s1
InChI 密鑰
RMCFMPMNMQZHSF-DHGKCCLASA-N
應用
β-D-Glucopyranosyl azide, 2-(acetylamino)-2-deoxy-, 3,4,6-triacetate is commonly used in organic synthesis, particularly for the preparation of glycosyl azides, which are versatile intermediates in the synthesis of glycoconjugates. In carbohydrate chemistry, it can be employed for modifying and functionalizing carbohydrates, including the introduction of azide groups. These azide-modified carbohydrates can participate in click chemistry reactions, enabling the development of bioconjugates and glycoarrays. In drug discovery, this compound aids in the design and synthesis of carbohydrate-based therapeutics, such as glycosidase inhibitors or glycoconjugate vaccines, by modifying carbohydrate structures. For biochemical research, this compound is valuable in studying carbohydrate-protein interactions, glycan biosynthesis, and glycoprotein engineering. It allows for the modification and labeling of carbohydrates for subsequent biological assays and analyses. Finally, as an azide derivative, it finds application in click chemistry reactions, specifically the azide-alkyne cycloaddition reaction (commonly known as the "click reaction"). This reaction enables efficient and selective labeling, bioconjugation, and cross-linking in various biological and materials science applications.
特點和優勢
β-D-Glucopyranosyl azide, 2-(acetylamino)-2-deoxy-, 3,4,6-triacetate is a complex carbohydrate and the glycosylation product of 2,3,4,6-tetraacetyl α--D--glucose and 2,3,6 -tri--O--acetyl--2--deoxy--β--D--glucopyranose. This compound has been modified by Click Chemistry with 4-(dimethylamino)pyridine (DMAP). The modification has produced an acetamido group at the C2 position of the glucopyranoside moiety. The compound is available in high purity for research purposes.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门