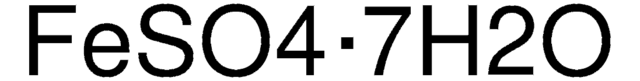推荐产品
品質等級
化驗
99.999% (trace metals basis)
形狀
crystalline
mp
117 °C
溶解度
H2O: soluble (highly soluble)
acetone: slightly soluble
alcohols: slightly soluble
SMILES 字串
O.O.O.O.O.O.Cl[Mg]Cl
InChI
1S/2ClH.Mg.6H2O/h2*1H;;6*1H2/q;;+2;;;;;;/p-2
InChI 密鑰
DHRRIBDTHFBPNG-UHFFFAOYSA-L
正在寻找类似产品? 访问 产品对比指南
一般說明
Magnesium chloride hexahydrate is a colorless to white, crystalline salt. The salt is soluble in water and slightly soluble in ethanol, acetone, and pyridine. The hydrate loses water when heated to higher temperatures, but thermal dehydration to the anhydrous salt is not straightforward. Chemically, magnesium chloride is a weak Lewis acid. One of the most common and simplest routes to producing magnesium chloride is extraction from naturally occurring brine, for example by solar evaporation of sea water, which can be purified by solution chemistries.
應用
One major application of high-purity magnesium chloride is in the synthesis of layered double hydroxides (LDH), which are high-surface area ionic clays. LDHs are studied for water filtration, among other applications, and usually consist of magnesium and aluminum ions because of the low toxicity of these metal cations. LDHs are synthesized from chloride salts using co-precipitation reactions, ion-exchange processes, and urea methods. Another application of high-purity magnesium chloride is as a reagent to dope or substitute magnesium ions into solid-state materials and nanoparticles. For example, Mg-doping in ZnO has shown to modulate its band structure and Mg-doping in lithium nickel cobalt manganese oxide helps suppress cation mixing and enables longer cycle lifetimes for its use as a cathode material.
儲存類別代碼
13 - Non Combustible Solids
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门