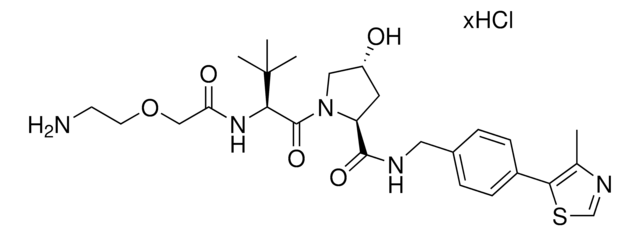
920851
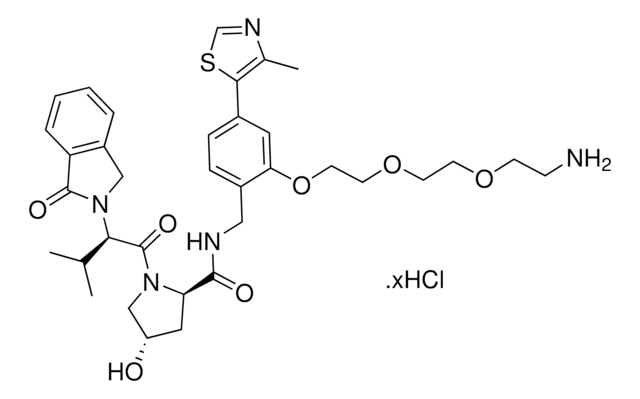
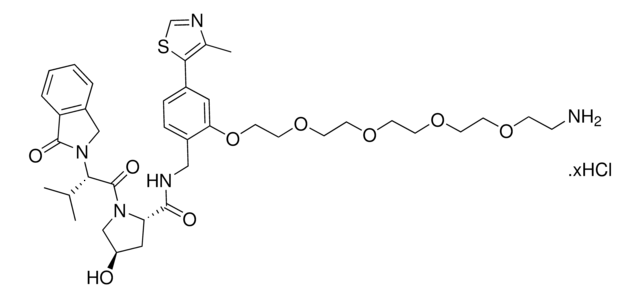
(S,R,S)-VL285 Phenol-C2-NH2 hydrochloride
别名:
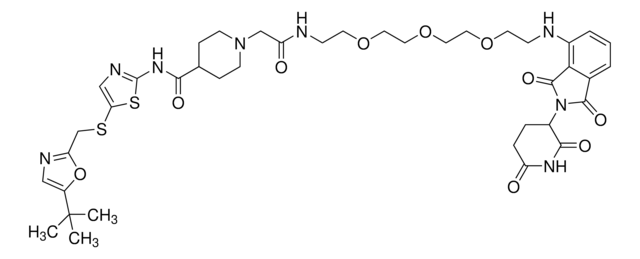
(2S,4R)-N-(2-(2-Aminoethoxy)-4-(4-methylthiazol-5-yl)benzyl)-4-hydroxy-1-((S)-3-methyl-2-(1-oxoisoindolin-2-yl)butanoyl)pyrrolidine-2-carboxamide hydrochloride, Crosslinker−E3 Ligase ligand conjugate, VHL protein degrader building block for PROTAC® research
登录查看公司和协议定价
所有图片(1)
About This Item
经验公式(希尔记法):
C31H37N5O5S · xHCl
分子量:
591.72 (free base basis)
NACRES:
NA.22
推荐产品
ligand
VL285 phenol
品質等級
形狀
solid
反應適用性
reactivity: carboxyl reactive
reagent type: ligand-linker conjugate
官能基
amine
儲存溫度
2-8°C
SMILES 字串
O=C1N([C@H](C(N(C2)[C@H](C(NCC3=CC=C(C=C3OCCN)C(SC=N4)=C4C)=O)C[C@H]2O)=O)C(C)C)CC5=C1C=CC=C5.Cl
應用
Protein degrader building block (S,R,S)-VL285 Phenol-C2-NH2 hydrochloride enables the synthesis of molecules for targeted protein degradation and PROTAC (proteolysis-targeting chimeras) technology. This conjugate contains a von Hippel-Lindau (VHL)-recruiting ligand with alternative exit vector from the widely used VH032 (901490), an alkyl-chain crosslinker, and a pendant amine for reactivity with a carboxylic acid on the target ligand. Because even slight alterations in ligands and crosslinkers can affect ternary complex formation between the target, E3 ligase, and PROTAC, many analogs are prepared to screen for optimal target degradation. When used with other protein degrader building blocks with a terminal amine, parallel synthesis can be used to more quickly generate PROTAC libraries that feature variation in crosslinker length, composition, and E3 ligase ligand.
其他說明
Technology Spotlight: Degrader Building Blocks for Targeted Protein Degradation
Portal: Building PROTAC® Degraders for Targeted Protein Degradation
Targeted Protein Degradation by Small Molecules
Targeted Protein Degradation: from Chemical Biology to Drug Discovery
HaloPROTACS: Use of Small Molecule PROTACs to Induce Degradation of HaloTag Fusion Proteins
Differential PROTAC substrate specificity dictated by orientation of recruited E3 ligase
Portal: Building PROTAC® Degraders for Targeted Protein Degradation
Targeted Protein Degradation by Small Molecules
Targeted Protein Degradation: from Chemical Biology to Drug Discovery
HaloPROTACS: Use of Small Molecule PROTACs to Induce Degradation of HaloTag Fusion Proteins
Differential PROTAC substrate specificity dictated by orientation of recruited E3 ligase
法律資訊
PROTAC is a registered trademark of Arvinas Operations, Inc., and is used under license
相關產品
产品编号
说明
价格
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Dennis L Buckley et al.
ACS chemical biology, 10(8), 1831-1837 (2015-06-13)
Small molecule-induced protein degradation is an attractive strategy for the development of chemical probes. One method for inducing targeted protein degradation involves the use of PROTACs, heterobifunctional molecules that can recruit specific E3 ligases to a desired protein of interest.
Daniel P Bondeson et al.
Annual review of pharmacology and toxicology, 57, 107-123 (2016-10-13)
Protein homeostasis networks are highly regulated systems responsible for maintaining the health and productivity of cells. Whereas therapeutics have been developed to disrupt protein homeostasis, more recently identified techniques have been used to repurpose homeostatic networks to effect degradation of
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门