推荐产品
形狀
powder or solid
分子量
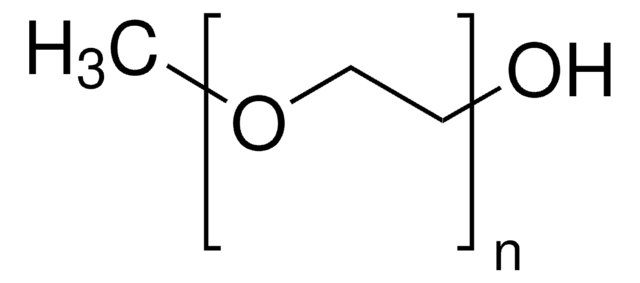
PEG average Mn 10,000
PEG ~10,000 Da
顏色
off-white to pale yellow
儲存溫度
2-8°C
一般說明
Poly(ethylene glycol) bis(2-pyridyl KAT) is a homobifunctional PEG featuring terminal potassium acyltrifluoroborate reactive groups for facile, rapid functionalization. Potassium acyltrifluoroborates (KATs) are stable functional groups that undergo rapid amide-forming ligations with hydroxylamines in aqueous media, in the presence of unprotected functional groups. In addition to its compatibility, these reactions proceed relatively quickly, lending to their use with sensitive biological reagents. This conjugation reaction offers a new approach to the synthesis of complex molecules without the complication of side reactions, such protein-polymer conjugates. KATs also undergo amide or imide-forming ligations in acidic conditions when reacted with primary amines or amides, respectively, as an alternative to classical acylation chemistry. Poly(ethylene glycol) bis(2-pyridyl KAT)s have been recently used in the rapid PEGylation and dimerization of expressed, folded protiens in near equimolar conditions, demonstrating the potential for these materials in a wide variety of drug delivery applications.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
PEGylation and Dimerization of Expressed Proteins under Near Equimolar Conditions with Potassium 2-Pyridyl Acyltrifluoroborates.
White CJ, et al.
ACS central science (2017)
Potassium Acyltrifluoroborate (KAT) Ligations are Orthogonal to Thiol-Michael and SPAAC Reactions: Covalent Dual Immobilization of Proteins onto Synthetic PEG Hydrogels.
Mazunin D, et al.
Helvetica Chimica Acta, 100(2) (2017)
Critical evaluation and rate constants of chemoselective ligation reactions for stoichiometric conjugations in water.
Saito, et al.
ACS Chemical Biology, 10, 1026-1033 (2015)
Alberto Osuna Gálvez et al.
Journal of the American Chemical Society, 139(5), 1826-1829 (2017-01-25)
Current methods for constructing amide bonds join amines and carboxylic acids by dehydrative couplings-processes that usually require organic solvents, expensive and often dangerous coupling reagents, and masking other functional groups. Here we describe an amide formation using primary amines and
Fumito Saito et al.
ACS chemical biology, 10(4), 1026-1033 (2015-01-13)
Chemoselective ligation reactions have contributed immensely to the development of organic synthesis and chemical biology. However, the ligation of stoichiometric amounts of large molecules for applications such as protein-protein conjugates is still challenging. Conjugation reactions need to be fast enough
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门
![聚 [2,6′]-4,8-二(5-乙基己基噻吩)苯并 [1,2-b;3,3-b] 二噻吩 ] {3-氟-2 [(2-乙基己基)羰基] 噻吩 [3,4-b] 噻吩二基 })](/deepweb/assets/sigmaaldrich/product/structures/187/203/ca94f947-403e-4832-8656-fc754d4148f5/640/ca94f947-403e-4832-8656-fc754d4148f5.png)