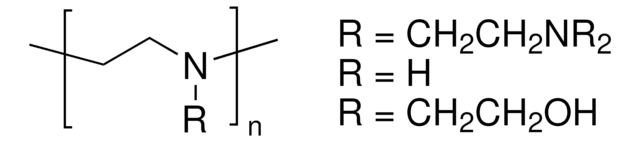推荐产品
品質等級
化驗
≥95%
形狀
powder or crystals
反應適用性
reagent type: catalyst
環保替代產品特色
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
環保替代類別
, Aligned
儲存溫度
−20°C
SMILES 字串
[H]C1([H])C([H])([H])O[C@@](C([H])([H])O[Si](C([H])([H])[H])(C([H])([H])[H])C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H])([H])[C@](O[H])([H])[C@]1([H])O[H]
InChI
1S/C12H26O4Si/c1-12(2,3)17(4,5)16-8-10-11(14)9(13)6-7-15-10/h9-11,13-14H,6-8H2,1-5H3/t9-,10-,11+/m1/s1
InChI 密鑰
KKFGQPZYDWCKRC-MXWKQRLJSA-N
一般說明
應用
其他說明
- Carbohydrate-Catalyzed Enantioselective Alkene Diboration:Enhanced Reactivity of 1,2-Bonded Diboron Complexes
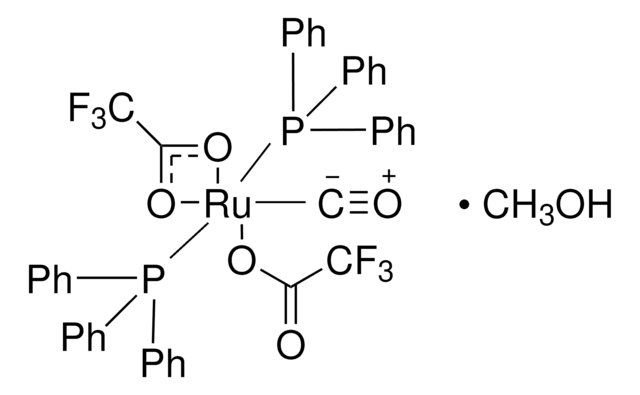
- Diols, α-Ketols, and Diones as 22π Components in [2+2+2] Cycloadditions of 1,6-Diynes via Ruthenium(0)-Catalyzed Transfer Hydrogenation
- Carbohydrate/DBU Cocatalyzed Alkene Diboration: Mechanistic Insight Provides Enhanced Catalytic Efficiency and Substrate Scope
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
商品
Enantioselective alkene diboration is a valuable strategy for transforming unsaturated hydrocarbons into useful chiral building blocks.
Enantioselective alkene diboration is a valuable strategy for transforming unsaturated hydrocarbons into useful chiral building blocks.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持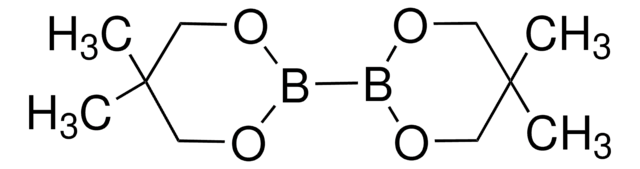
![1,8-二氮杂双环[5.4.0]十一碳-7-烯 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)
![Dichloro[9,9-dimethyl-4,5-bis(diphenylphosphino)xanthene]palladium(II) 95%](/deepweb/assets/sigmaaldrich/product/structures/374/597/f7932c5b-0448-498b-8254-f8ce1b9a4612/640/f7932c5b-0448-498b-8254-f8ce1b9a4612.png)