推荐产品
應用
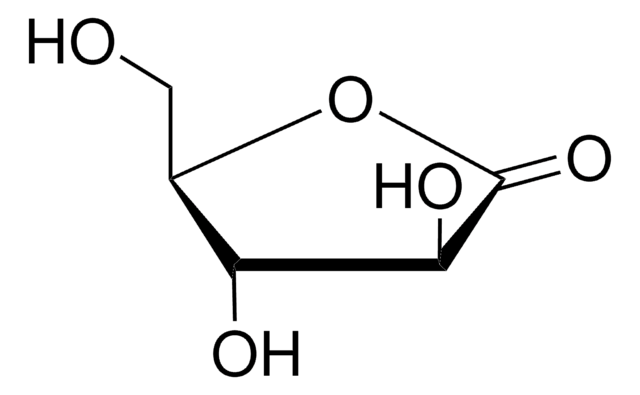
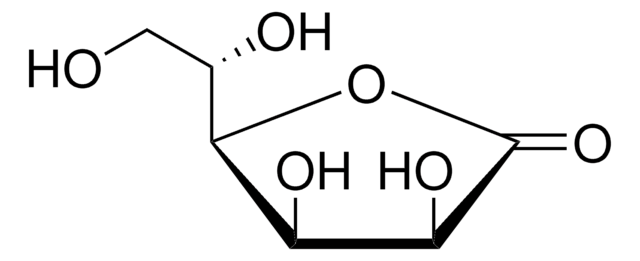
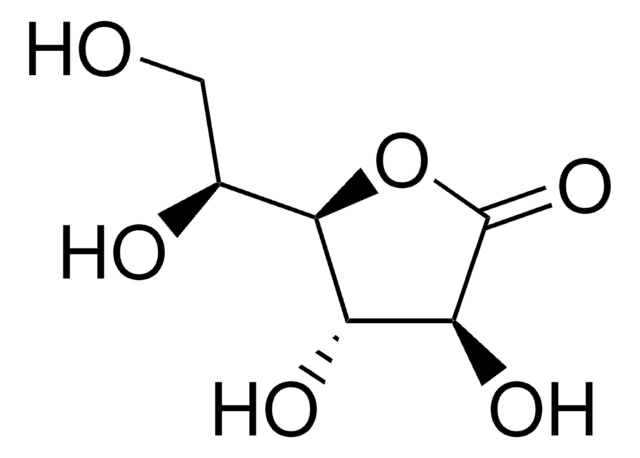
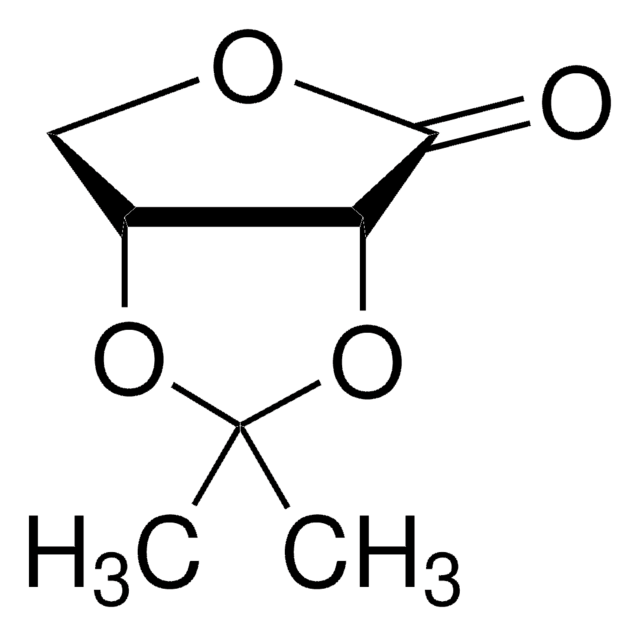
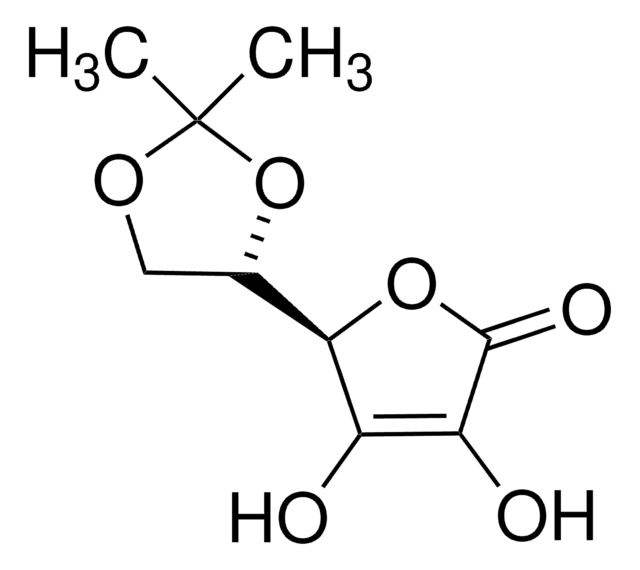
用于手性非环系、环戊烯酮类和氧杂双环体系的重要结构单元。还用于研究非线性光学材料。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
Chemistry of Materials, 5, 802-802 (1993)
Aldrichimica Acta, 22, 49-49 (1989)
Tae Woo Kim et al.
Organic letters, 6(22), 3949-3952 (2004-10-22)
[structure: see text] We describe a series of nonpolar nucleoside analogues having similar shapes and gradually increasing size. The structure of the nucleobase thymine was mimicked with toluene derivatives, replacing O2/O4 with hydrogen, fluorine, chlorine, bromine, and iodine. Glycosidic bonds
Cheng-Hung Jen et al.
Nucleosides, nucleotides & nucleic acids, 29(7), 523-534 (2010-07-01)
A thorough study for the synthesis of 1-deazauridine is described. 3-Bromo-2,6-dimethoxy-5-(beta-D-ribofuranosyl)pyridine, a synthetic precursor for 1-deazauridine, was prepared in seven steps from 2,6-dimethoxypyridine and d-ribose via the ribonolactone approach. Subsequent demethylation was unsuccessful but led to presumable anomerization and isomerization.
B A Horenstein et al.
Biochemistry, 32(28), 7089-7097 (1993-07-20)
A new approach to understanding transition-state structure is presented which involves the sequential application of experimental and computational methods. A family of experimentally determined kinetic isotope effects is fit simultaneously in a vibrational analysis to provide a geometric model of
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持