推荐产品
形狀
powder
品質等級
mp
156-161 °C
儲存溫度
2-8°C
SMILES 字串
O=S(N1C[C@H]2[P@@](C3=CC=C(OC)C=C3)C[C@@H]1C2)(C4=CC=C(C)C=C4)=O
InChI
1S/C19H22NO3PS/c1-14-3-9-19(10-4-14)25(21,22)20-12-18-11-15(20)13-24(18)17-7-5-16(23-2)6-8-17/h3-10,15,18H,11-13H2,1-2H3
InChI 密鑰
RMFZMNUGIFSNIE-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
The bicyclic, chiral phosphine was developed by the Kwon Research Group.Its initial applications were for asymmetric [3+2] annulation between allenes and imines as well as the first examples of phosphine-catalyzed asymmetric syntheses of 1,2,3,5-substituted pyrrolines. Along with nucleophilic organocatalysis, the P-chiral phosphines may also find utility in asymmetric transition-metal catalysis.
其他說明
Hydroxyproline-Derived Pseudoenantiomeric [2.2.1] Bicyclic Phosphines: Asymmetric Synthesis of (+)- and (-)-Pyrrolines
Technology Spotlight- Kwon Phosphines: P-Chiral Monodentate Phosphines from Hydroxyproline
Aldrichimica Acta Review- Nucleophilic Chiral Phosphines: Powerful and Versatile Catalysts for Asymmetric Annulations
Technology Spotlight- Kwon Phosphines: P-Chiral Monodentate Phosphines from Hydroxyproline
Aldrichimica Acta Review- Nucleophilic Chiral Phosphines: Powerful and Versatile Catalysts for Asymmetric Annulations
相關產品
产品编号
说明
价格
訊號詞
Warning
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Lingchao Cai et al.
Journal of the American Chemical Society, 138(10), 3298-3301 (2016-02-26)
Described herein is a catalytic asymmetric total synthesis of (-)-actinophyllic acid, with the key step being a chiral phosphine-catalyzed [3 + 2] annulation between an imine and an allenoate to form a pyrroline intermediate in 99% yield and 94% ee.
Yiting Gu et al.
Journal of the American Chemical Society, 137(19), 6400-6406 (2015-04-24)
Two classes of phosphine-catalyzed addition/cycloaddition domino reactions of β'-acetoxy allenoate 1 have been developed. The reaction of 1 with 2-acyl-3-methyl-acrylonitrile 2 readily occurs to give 2-oxabicyclo[3.3.1]nonane 3, furnishing the β'-addition/[4 + 4] cycloaddition domino sequence. In this sequence, β'C of
Ian P Andrews et al.
Chemical science, 3(8), 2510-2514 (2012-07-17)
In this study we performed the total synthesis of the terpene indole alkaloid (+)-ibophyllidine through a pathway involving asymmetric phosphine catalysis, with our novel l-4-hydroxyproline-derived chiral phosphine mediating the key [3 + 2] annulation. Hydrogenation of the [3 + 2]
Christopher E Henry et al.
Journal of the American Chemical Society, 136(34), 11890-11893 (2014-08-08)
We have prepared two new diastereoisomeric 2-aza-5-phosphabicyclo[2.2.1]heptanes from naturally occurring trans-4-hydroxy-L-proline in six chemical operations. These syntheses are concise and highly efficient, with straightforward purification. When we used these chiral phosphines as catalysts for reactions of γ-substituted allenoates with imines
商品
Chiral phosphines have been the staple ligands for asymmetric transition metal catalysis and more recently operate as catalysts in organic phosphinocatalysis.
Chiral phosphines have been the staple ligands for asymmetric transition metal catalysis and more recently operate as catalysts in organic phosphinocatalysis.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门![Exo-Phenyl Kwon [2.2.1]双环膦 95% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/477/026/5255f657-4af5-47da-9839-86b94d92129f/640/5255f657-4af5-47da-9839-86b94d92129f.png)
![Exo-4-anisole Kwon [2.2.1] bicyclic phosphine](/deepweb/assets/sigmaaldrich/product/structures/114/753/2a544671-b0e0-4556-8dc3-46c126d6c8ab/640/2a544671-b0e0-4556-8dc3-46c126d6c8ab.png)
![Endo-Phenyl Kwon [2.2.1] Bicyclic Phosphine 95% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/207/060/39f2b621-f484-49c3-b692-cdb610e8c517/640/39f2b621-f484-49c3-b692-cdb610e8c517.png)
![Exo-2-Naphthyl Kwon [2.2.1] Bicyclic Phosphine](/deepweb/assets/sigmaaldrich/product/structures/324/907/a6c29ce5-9be1-4585-9bfb-6dca8272f6e4/640/a6c29ce5-9be1-4585-9bfb-6dca8272f6e4.png)
![Endo-1-Naphthyl Kwon [2.2.1] Bicyclic Phosphine](/deepweb/assets/sigmaaldrich/product/structures/170/551/a39b471a-5427-43d5-9420-111b638ec1ac/640/a39b471a-5427-43d5-9420-111b638ec1ac.png)
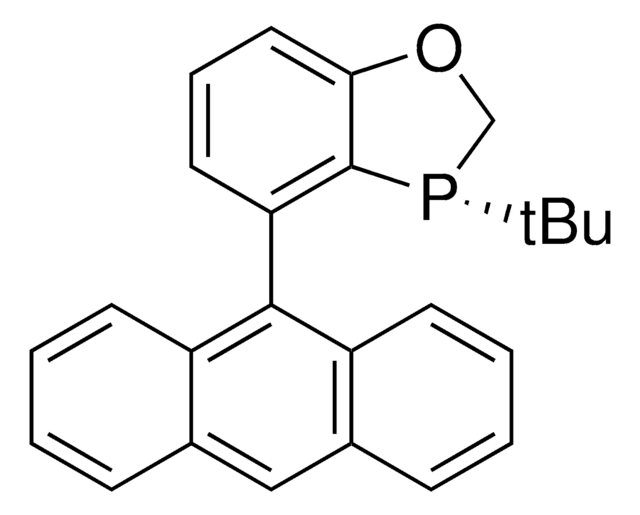
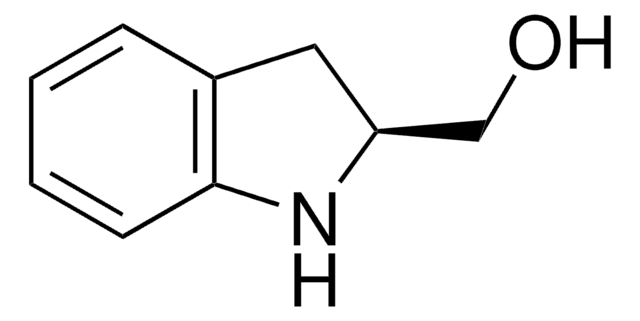
![(R)-(–)-4,12-双(二苯基膦)-[2.2]-对环芳烷 96%](/deepweb/assets/sigmaaldrich/product/structures/131/143/7e18cd49-a90e-4d89-a189-4f37ad9e6cd2/640/7e18cd49-a90e-4d89-a189-4f37ad9e6cd2.png)
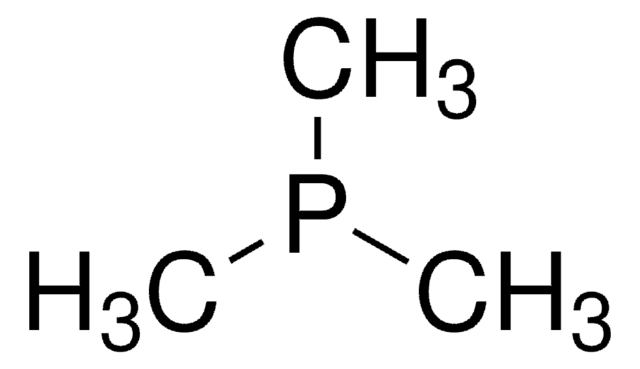
![Exo-1-Naphthyl Kwon [2.2.1] Bicyclic Phosphine](/deepweb/assets/sigmaaldrich/product/structures/376/476/5be457d0-df63-4a3d-823c-9a8002a7a813/640/5be457d0-df63-4a3d-823c-9a8002a7a813.png)
