About This Item
推荐产品
品質等級
化驗
≥95%
形狀
solid
mp
100-104 °C
官能基
phosphine
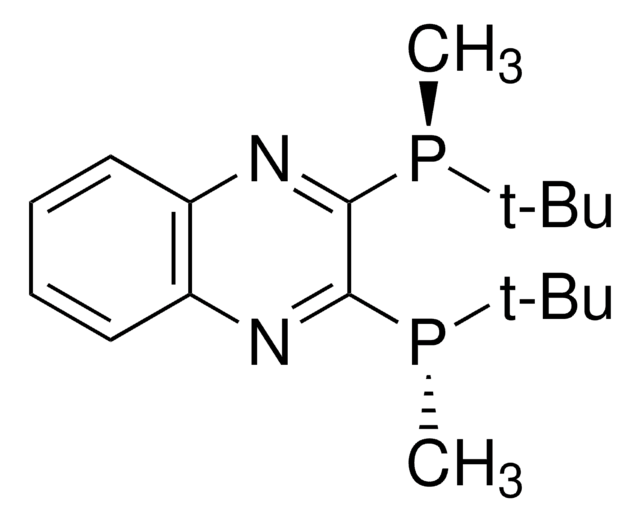
SMILES 字串
CP(c1nc2ccccc2nc1P(C)C(C)(C)C)C(C)(C)C
InChI
1S/C18H28N2P2/c1-17(2,3)21(7)15-16(22(8)18(4,5)6)20-14-12-10-9-11-13(14)19-15/h9-12H,1-8H3/t21-,22-/m0/s1
InChI 密鑰
DRZBLHZZDMCPGX-VXKWHMMOSA-N
一般說明
應用
特點和優勢
- It is not oxidized nor epimerized at ambient conditions in air
- Enantioselectivities are outstanding for various reaction paradigms
- Hydrogenations proceed under mild reaction conditions
- Low catalyst loadings yield high TONs
法律資訊
訊號詞
Danger
危險分類
Acute Tox. 3 Oral - Aquatic Chronic 4 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
商品
QuinoxP*: Air-Stable and Highly Efficient and Productive Chiral Ligands
QuinoxP*: Air-Stable and Highly Efficient and Productive Chiral Ligands
AEM3-944 | UAT | Prefill feature for related product categories not working
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持
![(R)-(-)-1-[(S)-2-二苯基磷]二茂铁乙基二环己基磷 ≥97%](/deepweb/assets/sigmaaldrich/product/structures/245/493/2ae2dd8a-65cc-4aba-9a1f-1292eb1ad8e0/640/2ae2dd8a-65cc-4aba-9a1f-1292eb1ad8e0.png)
![(+)-1,2-双[(2S,5S)-2,5-二甲基磷]苯 kanata purity](/deepweb/assets/sigmaaldrich/product/structures/319/912/cec7b70f-bf7c-4a96-9f11-a73ae892e34c/640/cec7b70f-bf7c-4a96-9f11-a73ae892e34c.png)