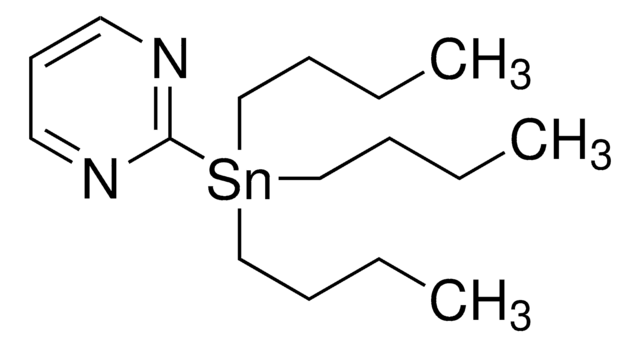
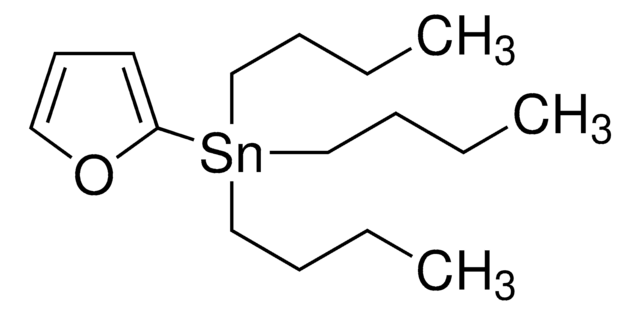
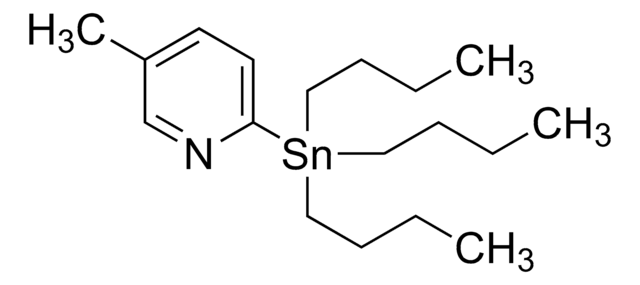
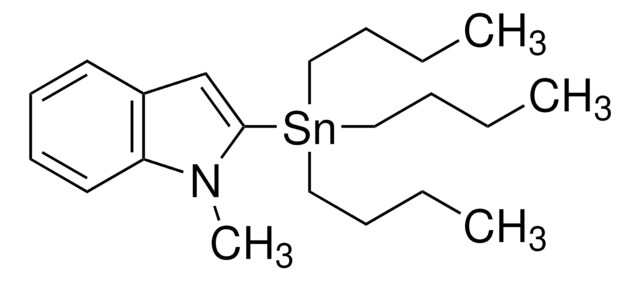
推荐产品
折射率
n20/D 1.4930 (lit.)
bp
108-110 °C/0.2 mmHg (lit.)
密度
1.170 g/mL at 25 °C
1.71 g/mL at 25 °C (lit.)
SMILES 字串
CCCC[Sn](CCCC)(CCCC)c1ncco1
InChI
1S/3C4H9.C3H2NO.Sn/c3*1-3-4-2;1-2-5-3-4-1;/h3*1,3-4H2,2H3;1-2H;
InChI 密鑰
YOWGRWHKDCHINP-UHFFFAOYSA-N
一般說明
2-(Tri-n-butylstannyl)oxazole is a synthetic building block used in Stille coupling reaction.
應用
2-(Tri-n-butylstannyl)oxazole can be used as a reactant to prepare:
- Heteroaromatic compounds via Stille-Migita cross-coupling reaction with (hetero)aryl halides using a palladium catalyst.
- Ethyl 2-[3-(1,3-oxazol-2-yl)-1H-indazol-1-yl]acetate by reacting with 3-iodoindazole in the presence of Pd(PPh3)4 as a catalyst.
訊號詞
Danger
危險分類
Acute Tox. 3 Oral - Acute Tox. 4 Dermal - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Repr. 1B - Skin Irrit. 2 - STOT RE 1
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Efficient synthesis of new 3-heteroaryl-1-functionalized 1H-indazoles
Fraile A, et al.
Tetrahedron, 67(1), 100-105 (2011)
Oliver Krebs et al.
Organic letters, 7(6), 1063-1066 (2005-03-12)
[structure: see text] A strategy for the synthesis of ajudazol A, an unusual, pharmacologically active metabolite from myxobacteria, based on the Stille cross-coupling of a 2-stannyl-oxazole with a vinyl iodide unit is described; the vinyl halide unit containing a (Z,Z)-diene
Jacob A Kaizerman et al.
Bioorganic & medicinal chemistry letters, 20(15), 4607-4610 (2010-07-03)
Pyridopyridazine antagonists of the hedgehog signaling pathway are described. Designed to optimize our previously described phthalazine smoothened antagonists, a representative compound eliminates a PXR liability while retaining potency and in vitro metabolic stability. Moreover, the compound has improved efficacy in
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门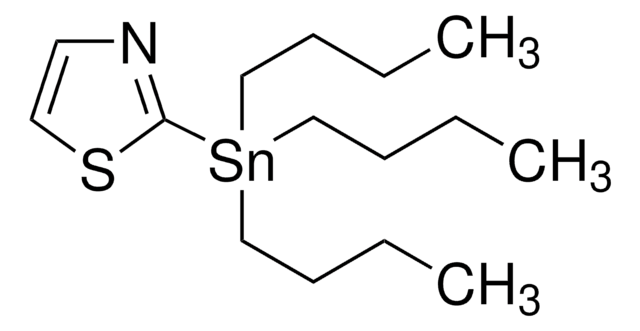

![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)二氯甲烷络合物](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)
![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)