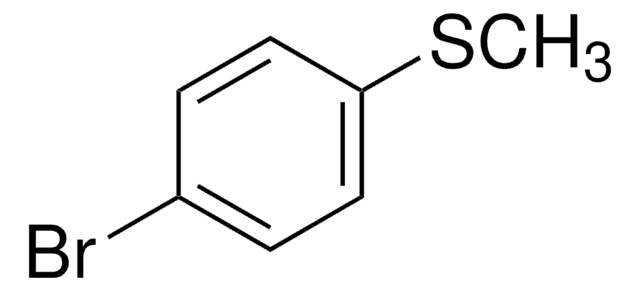
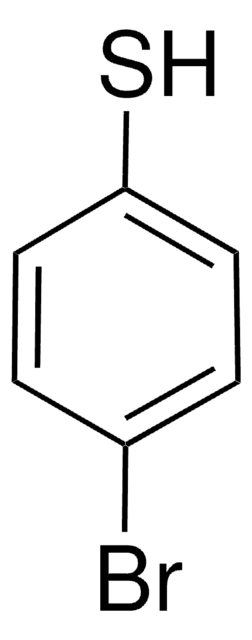
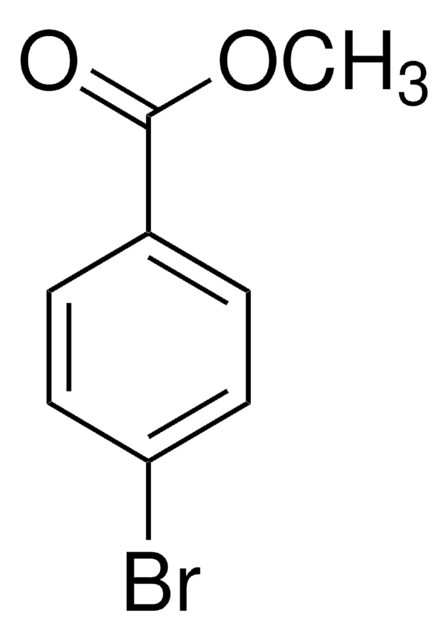
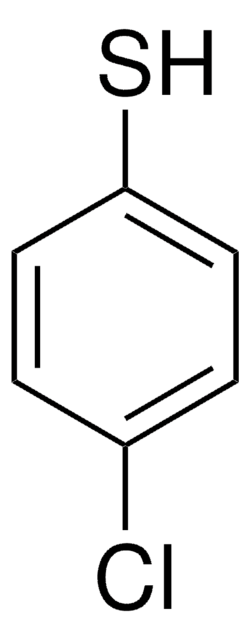
推荐产品
品質等級
化驗
97%
mp
103-107 °C (lit.)
SMILES 字串
CS(=O)(=O)c1ccc(Br)cc1
InChI
1S/C7H7BrO2S/c1-11(9,10)7-4-2-6(8)3-5-7/h2-5H,1H3
InChI 密鑰
FJLFSYRGFJDJMQ-UHFFFAOYSA-N
應用
4-Bromophenyl methyl sulfone may be used to synthesize:
4-Bromophenyl methyl sulfone (1-bromo-4-(methylsulfonyl)benzene) can undergo coupling reaction with benzene sulfonamide in the presence of copper(I)iodide to form the corresponding N-aryl sulfonamide.
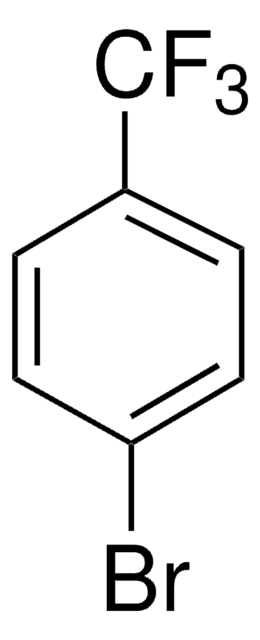
- biaryl methyl sulfones
- 5-[[-4-(methylsulfonyl)phenyl]thio]thiophene-2-sulfonamide
- 1-[4-(methylsulfonyl)phenyl]-1H-pyrazole (Hmsppz)
- DuP 697 via reaction with (5-chloro-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)thiophen-2-yl)trimethylsilane
4-Bromophenyl methyl sulfone (1-bromo-4-(methylsulfonyl)benzene) can undergo coupling reaction with benzene sulfonamide in the presence of copper(I)iodide to form the corresponding N-aryl sulfonamide.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
Cooperativity and steric hindrance: important factors in the binding of a-cyclodextrin with para-substituted aryl alkyl sulfides, sulfoxides and sulfones.
Davies DM and Deary ME.
J. Chem. Soc. Perkin Trans. II, 7, 1287-1294 (1995)
Daniel Tordera et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 19(26), 8597-8609 (2013-05-08)
A new approach to obtain green-emitting iridium(III) complexes is described. The synthetic approach consists of introducing a methylsulfone electron-withdrawing substituent into a 4-phenylpyrazole cyclometalating ligand in order to stabilize the highest-occupied molecular orbital (HOMO). Six new complexes have been synthesized
?Copper-catalyzed N-arylation of sulfonamides with aryl bromides under mild conditions?
Wang X, et al.
Tetrahedron Letters, 53, 7?10-7?10 (2012)
?Divergent synthesis of 2,3,5-substituted thiophenes by C-H activation/borylation/suzuki coupling?
Kallepalli.AV, et al.
Heterocycles, 80(2), 1429 - 1448 (2010)
Carol K Wada et al.
Journal of medicinal chemistry, 45(1), 219-232 (2002-01-05)
A novel series of sulfone N-formylhydroxylamines (retrohydroxamates) have been investigated as matrix metalloproteinases (MMP) inhibitors. The substitution of the ether linkage of ABT-770 (5) with a sulfone group 13a led to a substantial increase in activity against MMP-9 but was
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门