推荐产品
化驗
98%
mp
213-216 °C (lit.)
官能基
aldehyde
chloro
SMILES 字串
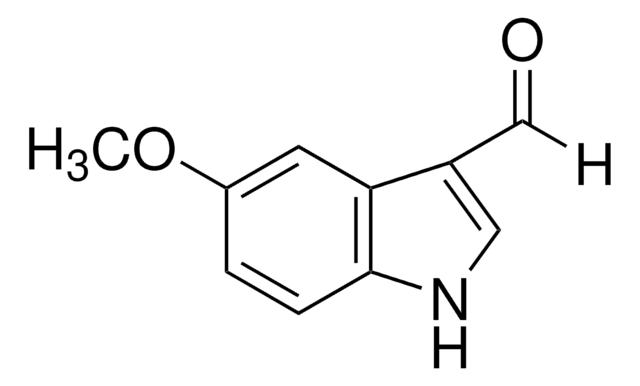
Clc1ccc2[nH]cc(C=O)c2c1
InChI
1S/C9H6ClNO/c10-7-1-2-9-8(3-7)6(5-12)4-11-9/h1-5,11H
InChI 密鑰
YXEXOIGXNYITQH-UHFFFAOYSA-N
一般說明
5-Chloroindole-3-carboxaldehyde, also known as 5-chloro-1H-indole-3-carboxaldehyde, is an indole derivative.
應用
5-Chloroindole-3-carboxaldehyde (5-Chloro-1H-indole-3-carboxaldehyde) may be used in the preparation of:
It may also be used in the preparation of the following hydrazone derivatives:
- 5-chloroindole-3-carboxaldehyde isonicotinoyl hydrazine
- 2′-[(5-chloro-1H-indol-3-yl)methylene]-2-(1H-indol-3-yl)acetohydrazide
- 5-chloro-3-(2,2-dibromovinyl)-1-(2-trimethylsilylethoxymethyl)indole
It may also be used in the preparation of the following hydrazone derivatives:
- 5-chloroindole-3-carboxaldehyde 3-chlorobenzoylhydrazone
- 5-chloroindole-3-carboxaldehyde 4-nitrobenzoylhydrazone
- 5-chloroindole-3-carboxaldehyde 3-methylbenzoylhydrazone
- 5-chloroindole-3-carboxaldehyde 4-methylbenzoylhydrazone
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Electrochemical behavior of indole-3-carboxaldehyde izonicotinoyl hydrazones: discussion on possible biological behavior
Shirinzadeh H, et al.
Combinatorial Chemistry & High Throughput Screening, 13(7), 619-627 (2010)
Tandem Suzuki-Miyaura cross-coupling/dehydrobromination of 1, 1-dibromoalkenes to alkynes with a cyclobutene-1, 2-diylbis (imidazolium) salt as catalyst precursor.
Rahimi A and Schmidt A.
Synthesis, 2010(15), 2621-2625 (2010)
2?-[(5-Chloro-1H-indol-3-yl) methylene]-2-(1H-indol-3-yl) acetohydrazide.
Ali HM, et al.
Acta Crystallographica Section E, Structure Reports Online, 63(4), o1807-o1808 (2007)
Kamaleddin Haj Mohammad Ebrahim Tehrani et al.
Iranian journal of pharmaceutical research : IJPR, 14(4), 1077-1086 (2015-12-15)
A series of indole-based aryl(aroyl)hydrazone analogs of antiplatelet indole-3-carboxaldehyde phenylhydrazone were synthesized by the Schiff base formation reaction and their antiplatelet activity was assessed using human platelet rich plasma. The platelet concentrate was obtained using a two-step centrifugation protocol and
Ming-Zhi Zhang et al.
European journal of medicinal chemistry, 92, 776-783 (2015-01-31)
Streptochlorin, first isolated as a new antibiotic in 1988 from the lipophilic extracts of the mycelium of a Streptomyces sp, is an indole natural products with a variety of biological activities. Based on the methods developed for the synthesis of
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门