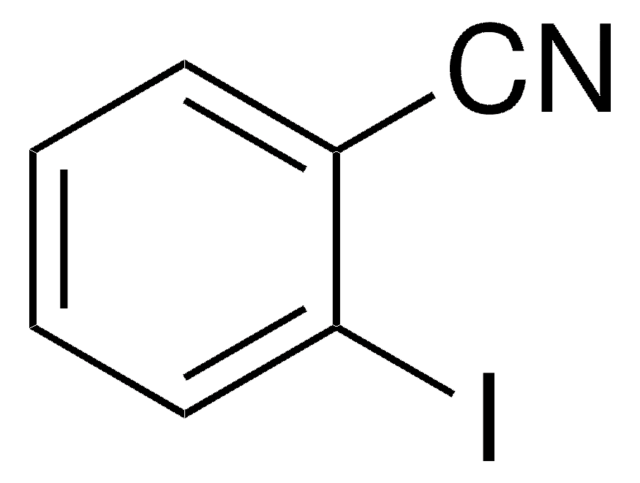
推荐产品
品質等級
化驗
98%
mp
40-43 °C (lit.)
官能基
iodo
nitrile
SMILES 字串
Ic1cccc(c1)C#N
InChI
1S/C7H4IN/c8-7-3-1-2-6(4-7)5-9/h1-4H
InChI 密鑰
BGARPMGQRREXLN-UHFFFAOYSA-N
一般說明
3-Iodobenzonitrile is a halogenated aromatic nitrile. Its standard (ρ° = 0.1MPa) molar enthalpy of formation was determined by combustion calorimetry.
應用
3-Iodobenzonitrile may be used as a starting reagent in the synthesis of tetrachloroisophthalo-[14C]-nitrile (TCIN). It may also be used in the preparation of:
- 1-(3-iodophenyl)-3-{2-[4-(trifluoromethyl)-1-piperidinyl]ethyl}-2-imidazolidinone
- piperidine derivative
- chiral amino acid anilide
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
230.0 °F - closed cup
閃點(°C)
110 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type N95 (US)
Thermodynamic and aromaticity studies for the assessment of the halogen? cyano interactions on Iodobenzonitrile.
Rocha IM, et al.
The Journal of Chemical Thermodynamics, 65, 204-212 (2013)
Synthesis of Chiral Amino Acid Anilides by Ligand-Free Copper-Catalyzed Selective N-Arylation of Amino Acid Amides
Dong J, et al.
Advanced Synthesis & Catalysis, 355(4), 692-696 (2013)
Idriss Bennacef et al.
Bioorganic & medicinal chemistry letters, 19(17), 5056-5059 (2009-07-29)
Compound 1 is a potent and selective antagonist of the dopamine D(3) receptor. With the aim of developing a carbon-11 labeled ligand for the dopamine D(3) receptor, 1 was selected as a potential PET probe. [(11)C]1 was obtained by palladium
Dominic P Affron et al.
European journal of organic chemistry, 2016(1), 139-149 (2016-02-16)
Saturated heterocycles, such as THFs, pyrrolidines, piperidines and THPs, are essential components of many biologically active compounds. Examples of C-H functionalization on these important ring systems remain scarce, especially at unactivated positions. Here we report the development of conditions for
Synthesis of tetrachloroisophthalo-[14C]-nitrile.
Davies PE.
Journal of Labelled Compounds & Radiopharmaceuticals, 21(3), 285-292 (1984)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门