所有图片(2)
About This Item
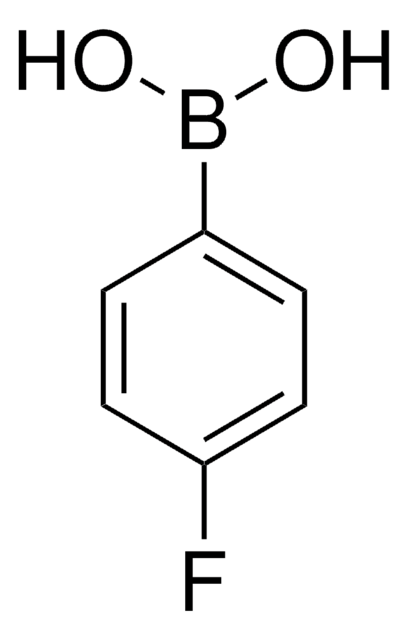
线性分子式:
C6H5OC6H4B(OH)2
CAS号:
分子量:
214.02
MDL號碼:
分類程式碼代碼:
12352103
PubChem物質ID:
NACRES:
NA.22
推荐产品
品質等級
化驗
≥95.0%
mp
141-145 °C (lit.)
官能基
phenoxy
SMILES 字串
OB(O)c1ccc(Oc2ccccc2)cc1
InChI
1S/C12H11BO3/c14-13(15)10-6-8-12(9-7-10)16-11-4-2-1-3-5-11/h1-9,14-15H
InChI 密鑰
KFXUHRXGLWUOJT-UHFFFAOYSA-N
應用
4-Phenoxyphenylboronic acid can be used as a reactant:
- In the Suzuki-Miyaura coupling reaction to synthesize aryl derivatives via C-C bond formation by reacting with different aryl halides over a palladium catalyst.
- To prepare 1-phenoxy-4-(trifluoromethyl)benzene via oxidative trifluoromethylation using Chan-Lam-type reaction conditions.
- To synthesize evobrutinib, a potent Bruton′s tyrosine kinase (BTK) inhibitor.
其他說明
含有不定量的酸酐
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
Room temperature aryl trifluoromethylation via copper-mediated oxidative cross-coupling
Senecal TD, et al.
The Journal of Organic Chemistry, 76(4), 1174-1176 (2011)
Efficacy and pharmacodynamic modeling of the BTK inhibitor evobrutinib in autoimmune disease models
Haselmayer P, et al.
Journal of Immunology, 202(10), 2888-2906 (2019)
Global Trade Item Number
| 货号 | GTIN |
|---|---|
| 480142-25G | 4061832710709 |
| 480142-5G | 4061832710716 |
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持