推荐产品
化驗
97%
折射率
n20/D 1.561 (lit.)
密度
1.024 g/mL at 25 °C (lit.)
SMILES 字串
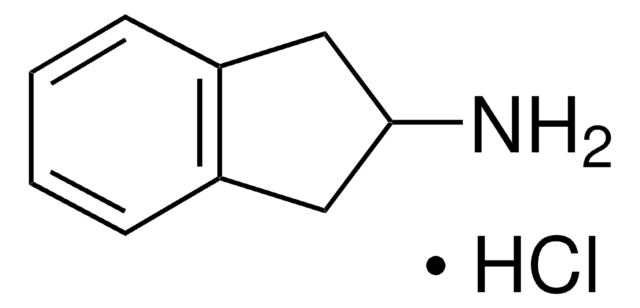
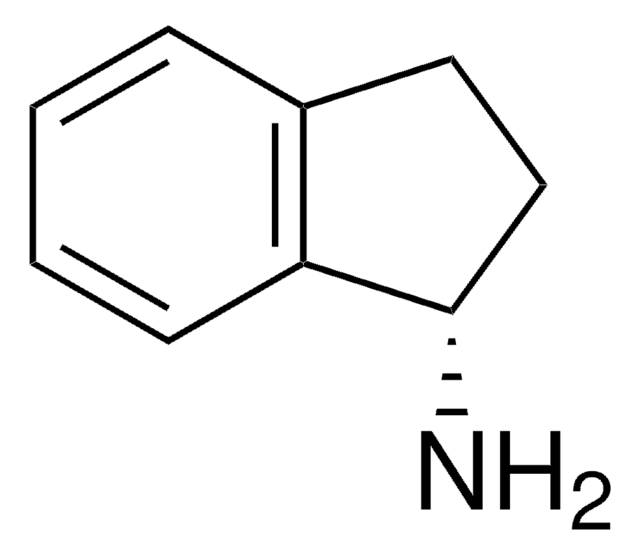
NC1Cc2ccccc2C1
InChI
1S/C9H11N/c10-9-5-7-3-1-2-4-8(7)6-9/h1-4,9H,5-6,10H2
InChI 密鑰
LMHHFZAXSANGGM-UHFFFAOYSA-N
一般說明
2-Aminoindan (2-Aminoindane) is an analog of amphetamine. It shows a potential bronchodilator and analgesic effect. The impact of the intramolecular N-H···Π hydrogen bonding on the conformations of 2-Al has been analyzed.
應用
2-Aminoindan may be used to prepare trans-(1S,2S)-2-amino-1-indanol via hydroxylation using dopamine β-hydroxylase (DBH) enzyme. It may be used to synthesize 2-amino-4-chloro-6-(2-aminoindanyl)-1,3,5-triazine.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Adam L Halberstadt et al.
Psychopharmacology, 236(3), 989-999 (2019-03-25)
Over the last decade, many new psychostimulant analogues have appeared on the recreational drug market and most are derivatives of amphetamine or cathinone. Another class of designer drugs is derived from the 2-aminoindan structural template. Several members of this class
Hiroshi Iga et al.
The journal of physical chemistry. A, 111(27), 5981-5987 (2007-06-19)
Laser-induced fluorescence (LIF), dispersed fluorescence (DF), mass-resolved one-color resonance enhanced two-photon ionization (RE2PI) and UV-UV hole-burning spectra of 2-aminoindan (2-AI) were measured in a supersonic jet. The hole-burning spectra demonstrated that the congested vibronic structures observed in the LIF excitation
Enzymatic Hydroxylation by Dopamine β-Hydroxylase.
Mitrochkine AA, et al.
European Journal of Organic Chemistry, 1998(6), 1171-1176 (1998)
J G Cannon et al.
Journal of medicinal chemistry, 23(7), 745-749 (1980-07-01)
Three series of bicyclic, semirigid congeners of beta-phenethylamine have been prepared for evaluation of the effect of ring size (and of concomitant conformational variation) on biological activity in a variety of assays for adrenergic and dopaminergic actions. Pharmacologic activity was
P J Hajduk et al.
Journal of medicinal chemistry, 42(19), 3852-3859 (1999-10-03)
The Erm family of methyltransferases confers resistance to the macrolide-lincosamide-streptogramin type B (MLS) antibiotics through the methylation of 23S ribosomal RNA. Upon the methylation of RNA, the MLS antibiotics lose their ability to bind to the ribosome and exhibit their
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门