所有图片(2)
About This Item
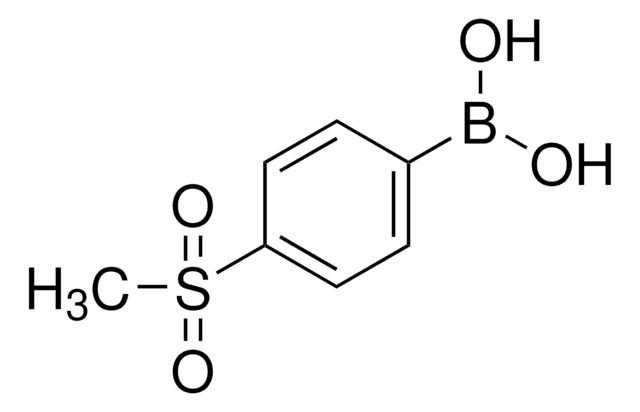
线性分子式:
CH3COC6H4B(OH)2
CAS号:
分子量:
163.97
MDL號碼:
分類程式碼代碼:
12352103
PubChem物質ID:
NACRES:
NA.22
推荐产品
品質等級
化驗
95%
mp
240-244 °C (lit.)
官能基
ketone
SMILES 字串
CC(=O)c1ccc(cc1)B(O)O
InChI
1S/C8H9BO3/c1-6(10)7-2-4-8(5-3-7)9(11)12/h2-5,11-12H,1H3
InChI 密鑰
OBQRODBYVNIZJU-UHFFFAOYSA-N
一般說明
4-Acetylphenylboronic acid is a boronate, belongs to a class of synthetic organic compounds. It reacts rapidly with peroxynitrite (ONOO(-)) to form stable hydroxy derivatives. It undergoes Suzuki coupling with 4-bromotriphenylamine catalyzed by dichlorobis(triphenylphosphine)Pd(II), during the synthesis of dendrimers.
應用
4-Acetylphenylboronic acid was used in the synthesis of 4′-azidoacetophenone.
Reactant involved in:
- Palladium-catalyzed decarboxylative coupling
- Copper-catalyzed hydroxylation
- Palladium-catalyzed Suzuki-Miyaura cross-coupling
- Cross-coupling with α-bromocarbonyl compounds
- Oxidation catalyzed by Baeyer-Villiger monooxygenases
- 1,5-substitution reactions
其他說明
含有不定量的酸酐
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Kimberly D Grimes et al.
Synthesis, 2010(9), 1441-1448 (2010-06-08)
We report the copper(II)-catalyzed conversion of organoboron compounds into the corresponding azide derivatives. A systematic series of phenylboronic acid derivatives is evaluated to examine the importance of steric and electronic effects of the substituents on reaction yield as well as
Adam Sikora et al.
Free radical biology & medicine, 47(10), 1401-1407 (2009-08-19)
In this study, we show that boronates, a class of synthetic organic compounds, react rapidly and stoichiometrically with peroxynitrite (ONOO(-)) to form stable hydroxy derivatives as major products. Using a stopped-flow kinetic technique, we measured the second-order rate constants for
A New Efficient Convergent Synthesis of Conjugated Aryl-containing Dendrimers.
El-Deeb IM and Lee SH.
Bull. Korean Chem. Soc., 31(6), 1757-1760 (2010)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门![1-[4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]ethanone AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/280/787/64aa2a50-1d44-4c16-ace9-c54ea40606e6/640/64aa2a50-1d44-4c16-ace9-c54ea40606e6.png)