所有图片(1)
About This Item
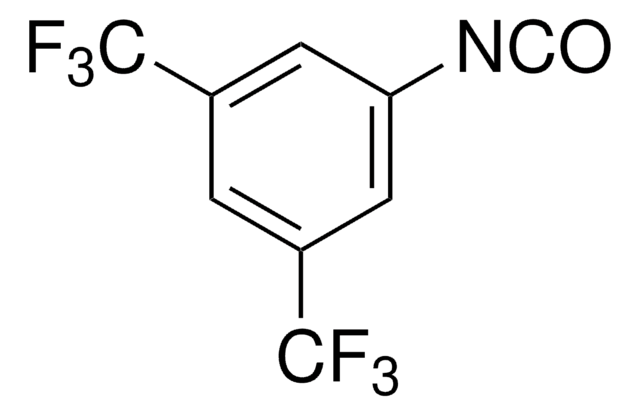
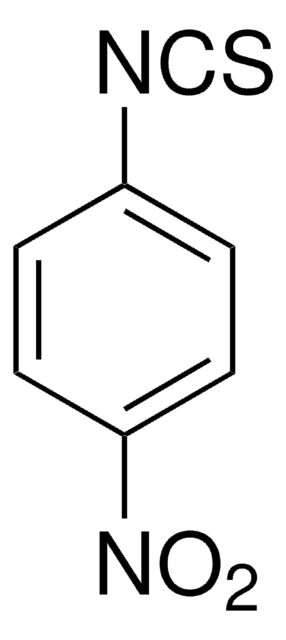
线性分子式:
(CF3)2C6H3NCS
CAS号:
分子量:
271.18
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
品質等級
化驗
98%
折射率
n20/D 1.5 (lit.)
bp
63 °C/1.5 mmHg (lit.)
密度
1.485 g/mL at 25 °C (lit.)
官能基
fluoro
isothiocyanate
SMILES 字串
FC(F)(F)c1cc(cc(c1)C(F)(F)F)N=C=S
InChI
1S/C9H3F6NS/c10-8(11,12)5-1-6(9(13,14)15)3-7(2-5)16-4-17/h1-3H
InChI 密鑰
FXOSSGVJGGNASE-UHFFFAOYSA-N
應用
3,5-双(三氟甲基)苯基异硫氰酸酯已用于以下研究:
- 氨基官能化模型表面的化学衍生化,金上的氨基硫醇盐,硅上的氨基硅氧烷,以及聚乙烯(PE)箔和膜。
- ω-氨基-4,4′-三联苯基取代的链烷硫醇的自组装单层的化学衍生化。
- 乙烯基磺酰亚胺硫脲的合成。
- 吡咯烷-2-羧酸-{2- [3-(3,5-双三氟甲基苯基)硫脲基]苯基}-酰胺的制备。
訊號詞
Danger
危險分類
Acute Tox. 3 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
228.2 °F - closed cup
閃點(°C)
109 °C - closed cup
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
其他客户在看
Paul M Dietrich et al.
Langmuir : the ACS journal of surfaces and colloids, 26(6), 3949-3954 (2010-01-01)
Amino-terminated self-assembled monolayers on gold substrates were studied by X-ray photoelectron spectroscopy (XPS), near-edge X-ray absorption fine structure (NEXAFS) measurements, and atomic force microscopy (AFM). Two different omega-amino-4,4'-terphenyl substituted alkanethiols of the general structure H(2)N-(C(6)H(4))(3)-(CH(2))(n)-SH (ATPn) were used: 2-(4''-amino-1,1':4',1''-terphenyl-4-yl)ethane-1-thiol (n
Highly diastereo-and enantioselective direct aldol reactions promoted by water-compatible organocatalysts bearing a pyrrolidinyl-camphor structural scaffold.
Tzeng Z-H, et al.
Tetrahedron, 65(5), 2879-2888 (2009)
Synthesis of chiral sulfoximine-based thioureas and their application in asymmetric organocatalysis.
Marcus Frings et al.
Beilstein journal of organic chemistry, 8, 1443-1451 (2012-09-29)
For the first time, chiral sulfoximine derivatives have been applied as asymmetric organocatalysts. In combination with a thiourea-type backbone the sulfonimidoyl moiety leads to organocatalysts showing good reactivity in the catalytic desymmetrization of a cyclic meso-anhydride and moderate enantioselectivity in
Nora Graf et al.
Analytical and bioanalytical chemistry, 396(2), 725-738 (2009-11-06)
The determination of amino groups on surfaces capable of binding biomolecules is important for the understanding and optimization of technologically relevant coupling processes. In this study, three different types of amino-functionalized model surfaces, amino thiolate on Au, amino siloxane on
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持