推荐产品
蒸汽壓力
12.46 psi ( 55 °C)
3.6 psi ( 20 °C)
品質等級
形狀
liquid
濃度
1.0 M in THF
密度
0.927 g/mL at 25 °C
儲存溫度
2-8°C
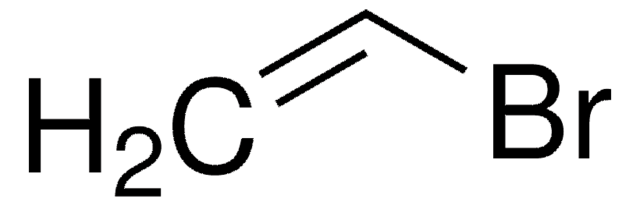
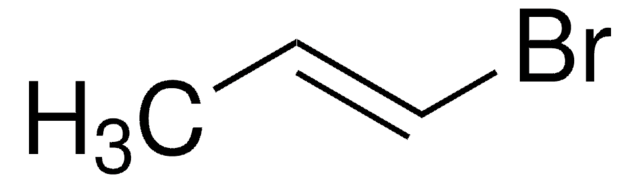
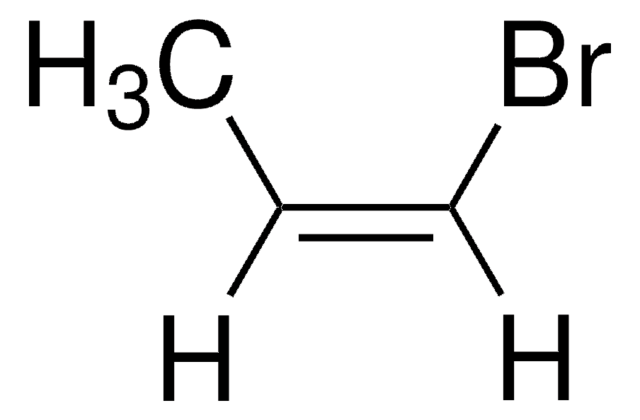
SMILES 字串
BrC=C
InChI
1S/C2H3Br/c1-2-3/h2H,1H2
InChI 密鑰
INLLPKCGLOXCIV-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
乙烯基溴属于卤代烯 ,由于未饱和乙烯基的存在,具有很高的反应活性。在加入聚合物或材料的结构中时,它可以为聚合物或材料带来阻燃性。它也是一种多功能的结构单元,可用于聚合、加成反应、取代反应和交联反应,如Suzuki-Miyaura 和Negishi反应。 它可用于将放射性标记引入医学成像用分子。
應用
- 在电子转移介质存在下,乙烯基溴的电化学还原对二氧化碳进行序贯乙烯基环化/固定:本研究探讨了乙烯基溴的电化学还原,重点关注乙烯基自由基环化和二氧化碳固定(A Katayama, H Senboku, 2016)。
- 氩气基质中臭氧与溴乙烯和氟化物波长相关光化学反应的比较:该研究比较了乙烯基溴和氟化物与臭氧的光化学反应,考察了它们在氩气基质中的特性(BS Ault, 2021)。
乙烯基溴溶液可用作前体,用于通过不对称还原偶联,进行手性2-乙烯基四氢萘的立体选择性合成。这些手性化合物是天然产物、农用化学品和液晶中的重要结构单元。
訊號詞
Danger
危險分類
Acute Tox. 4 Oral - Carc. 1B - Eye Irrit. 2 - Flam. Liq. 2 - STOT SE 3
標靶器官
Central nervous system, Respiratory system
安全危害
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
1.4 °F - closed cup
閃點(°C)
-17 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
Changhui Sun et al.
Organic letters, 11(18), 4084-4087 (2009-08-20)
With the catalysis of CuI/trans-N,N'-dimethylcyclohexane-1,2-diamine, a number of carboxylic acids underwent efficient intramolecular O-vinylation with vinyl bromides leading to the synthesis of the corresponding five- and six-membered enol lactones. The same catalytic system also led to the efficient cycloisomerization of
Qiwu Zhao et al.
Organic letters, 10(18), 4037-4040 (2008-08-30)
A general and highly efficient synthesis of 4-alkylidene-2-azetidinones was achieved by the Cu(I)-catalyzed intramolecular C-N coupling of amides with vinyl bromides. This 4-exo ring closure was found to be fundamentally preferred over other modes (5-exo, 6-exo, and 6-endo) of cyclization
Derek R Boyd et al.
Organic & biomolecular chemistry, 5(3), 514-522 (2007-01-26)
Enantiopure trans-dihydrodiols have been obtained by a chemoenzymatic synthesis from the corresponding cis-dihydrodiol metabolites, obtained by dioxygenase-catalysed arene cis-dihydroxylation at the 2,3-bond of monosubstituted benzene substrates. This generally applicable, seven-step synthetic route to trans-dihydrodiols involves a regioselective hydrogenation and a
Ian Paterson et al.
Organic letters, 12(16), 3724-3727 (2010-08-14)
Using a combination of asymmetric vinylogous Mukaiyama aldol and Stille cross-coupling reactions, an advanced polyene fragment of the chivosazoles was prepared in a highly stereocontrolled manner. This key C1-C13 pentaene subunit, featuring the conjugated (2E,4Z,6E,8Z)-tetraenoate motif and anti-configured C10 and
Carcinogenicity of 1,3-butadiene, ethylene oxide, vinyl chloride, vinyl fluoride, and vinyl bromide.
Yann Grosse et al.
The Lancet. Oncology, 8(8), 679-680 (2007-08-30)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持