所有图片(1)
About This Item
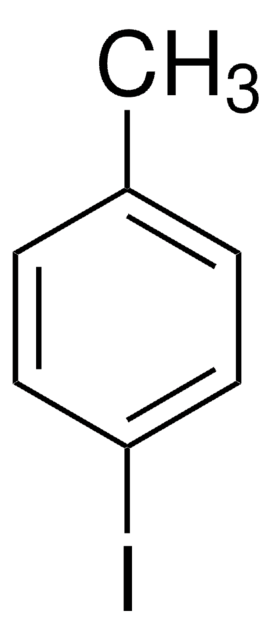
线性分子式:
(CH3)2C6H3I
CAS号:
分子量:
232.06
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
品質等級
化驗
99%
形狀
liquid
折射率
n20/D 1.594 (lit.)
bp
92-94 °C/3 mmHg (lit.)
密度
1.608 g/mL at 25 °C (lit.)
官能基
iodo
SMILES 字串
Cc1cc(C)cc(I)c1
InChI
1S/C8H9I/c1-6-3-7(2)5-8(9)4-6/h3-5H,1-2H3
InChI 密鑰
ZLMKEENUYIUKKC-UHFFFAOYSA-N
一般說明
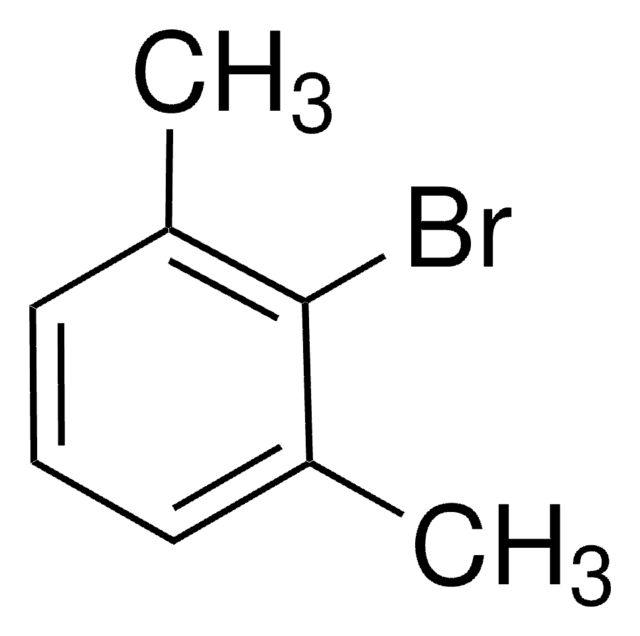
1-Iodo-3,5-dimethylbenzene (5-Iodo-m-xylene) is an aryl halide. It can be obtained from 5-bromo-m-xylene, via copper-catalyzed halogen exchange reaction, in the presence of NaI or KI in n-BuOH or DMF (solvents). It undergoes reaction with phenol in the presence of CuFe2O4 nano powder as a recyclable catalyst to afford 1,3-dimethyl-5-phenoxybenzene.
應用
1-Iodo-3,5-dimethylbenzene (5-iodo-m-xylene) is suitable for use in the synthesis of N-(3,5-xylyl)-N-ethylaniline, an arylamine.
It may be used in the following studies:
It may be used in the following studies:
- α-Arylation of ketones.
- Copper-catalyzed N-arylation of imidazoles.
- Cyanation of 5-iodo-m-xylene to form 3,5-dimethylbenzonitrile.
- Synthesis of 1,3-Dimethyl-5-phenoxybenzene by nano-CuFe2O4 catalyzed C-O cross-coupling with phenol.
- CuBr-catalyzed amination of 1-iodo-3,5-dimethylbenzene to form N-Allyl-3,5-dimethylbenzenamine.
- Copper-catalyzed C-S bond-formation between 5-iodo-m-xylene and thiophenol.
- As a starting material in the synthesis of biphenyl-3,3′,5,5′-tetracarboxylic acid.
- Radical bromination of 5-iodo-m-xylene by N-bromosuccinimide to form 1,3-bis(bromomethyl)-5-iodobenzene.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
235.4 °F - closed cup
閃點(°C)
113 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
Anouk S Lubbe et al.
The Journal of organic chemistry, 76(21), 8599-8610 (2011-09-21)
A study is presented on the control of rotary motion of an appending rotor unit in a light-driven molecular motor. Two new light driven molecular motors were synthesized that contain aryl groups connected to the stereogenic centers. The aryl groups
Fuk Yee Kwong et al.
Organic letters, 4(20), 3517-3520 (2002-09-27)
An efficient copper-catalyzed carbon-sulfur bond formation reaction was developed. This method is particularly noteworthy given its experimental simplicity, high generality, and exceptional level of functional group toleration and the low cost of the catalyst system. [reaction: see text]
On the synthesis of heterocyclic dendrons.
Diez-Barra E, et al.
ARKIVOC (Gainesville, FL, United States), 2002(5), 17-25 (2002)
Recyclable and reusable nano-CuFe2O4 catalyzed CO cross-coupling.
Avudoddi V, et al.
European Journal of Chemistry, 3(3), 298-304 (2012)
Recyclable and reusable nano-CuFe2O4 catalyzed CO cross-coupling.
Avudoddi V, et al.
European Journal of Organic Chemistry, 3(3), 298-304 (2012)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持