所有图片(3)
About This Item
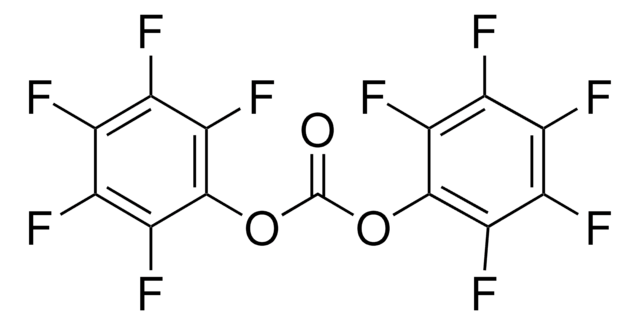
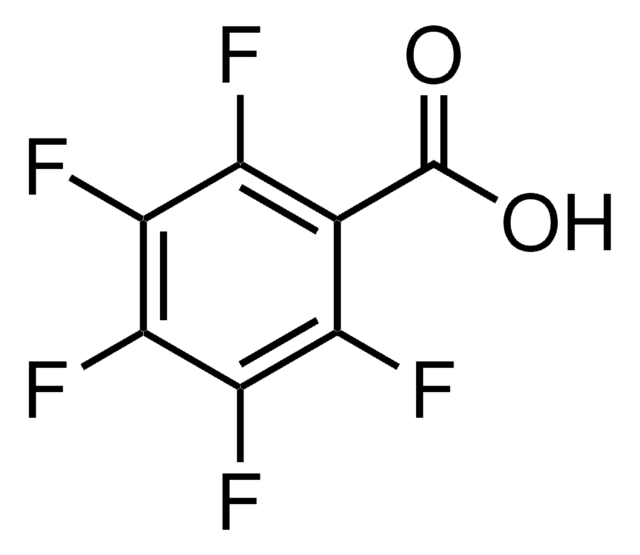
线性分子式:
CF3CO2C6F5
CAS号:
分子量:
280.07
Beilstein:
2003848
MDL號碼:
分類程式碼代碼:
12352101
PubChem物質ID:
NACRES:
NA.22
推荐产品
product name
三氟乙酸五氟苯酯, 98%
品質等級
化驗
98%
形狀
liquid
折射率
n20/D 1.368 (lit.)
bp
122-123 °C (lit.)
密度
1.63 g/mL at 25 °C (lit.)
應用
peptide synthesis
官能基
ester
fluoro
SMILES 字串
Fc1c(F)c(F)c(OC(=O)C(F)(F)F)c(F)c1F
InChI
1S/C8F8O2/c9-1-2(10)4(12)6(5(13)3(1)11)18-7(17)8(14,15)16
InChI 密鑰
VCQURUZYYSOUHP-UHFFFAOYSA-N
應用
三氟乙酸五氟苯酯可用于:
- 2′-羧基罗丹明的酯交换反应以形成单一异构体五氟苯酯
- 作为酰化剂和偶联剂用于N-取代甘氨酸低聚物的肽型偶联
- 通过与3-噻吩乙酸反应合成五氟苯基噻吩-3-乙酸酯 (PFPTA)。
其他說明
可能含有少量的五氟苯酚
訊號詞
Warning
危險分類
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
125.6 °F - closed cup
閃點(°C)
52 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
Unexpected reactivity of the 2′-carboxyl functionality in rhodamine dyes.
Haack RA, et al.
Tetrahedron Letters, 58(18), 1733-1737 (2017)
Weak backbone CH?O=C and side chain t Bu?t Bu London interactions help promote helix folding of achiral NtBu peptoids.
Angelici G, et al.
Chemical Communications (Cambridge, England), 52(24), 4573-4576 (2016)
Vancomycin-conjugated polythiophene for the detection and imaging of Gram-positive bacteria.
Ning LG, et al.
Journal of Material Chemistry B: Materials for Biology and Medicine, 5(44), 8814-8820 (2017)
Nathan J Van Zee et al.
Nature, 558(7708), 100-103 (2018-06-01)
Water directs the self-assembly of both natural1,2 and synthetic3-9 molecules to form precise yet dynamic structures. Nevertheless, our molecular understanding of the role of water in such systems is incomplete, which represents a fundamental constraint in the development of supramolecular
Rory K Morgan et al.
ACS chemical biology, 10(8), 1778-1784 (2015-05-16)
ADP-ribosylation is essential for cell function, yet there is a dearth of methods for detecting this post-translational modification in cells. Here, we describe a clickable aminooxy alkyne (AO-alkyne) probe that can detect cellular ADP-ribosylation on acidic amino acids following Cu-catalyzed
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门