所有图片(1)
About This Item
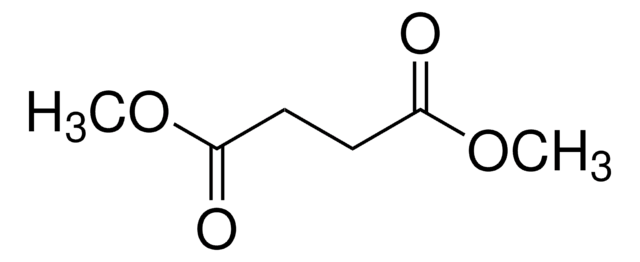
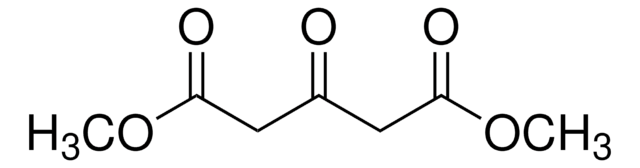
线性分子式:
CH3O2CCH2CH2COCO2CH3
CAS号:
分子量:
174.15
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
96%
形狀
liquid
折射率
n20/D 1.439 (lit.)
bp
90-95 °C/0.4 mmHg (lit.)
密度
1.203 g/mL at 25 °C (lit.)
官能基
ester
ketone
SMILES 字串
COC(=O)CCC(=O)C(=O)OC
InChI
1S/C7H10O5/c1-11-6(9)4-3-5(8)7(10)12-2/h3-4H2,1-2H3
InChI 密鑰
TXIXSLPEABAEHP-UHFFFAOYSA-N
一般說明
2-氧戊二酸二甲酯是在Krebs循环期间形成的关键中间体,并且是生物代谢途径中的重要氮转运子。2-氧戊二酸二甲酯的电化学行为已通过循环伏安法、方波伏安法和使用玻碳电极的差分脉冲伏安法进行了研究。
應用
2-氧代戊二酸二甲酯可以与双亲核物,如1,2-苯二胺、2-氨基苯酚和2-氨基苯硫醇,进行环缩合以形成新的杂环。
2-氧戊二酸二甲酯可用于合成构象受限的PNA(肽核酸)单体,该单体能够在三重基序中结合胸腺嘧啶。它可以通过与硝基苯乙烯的高度立体选择性Michael加成反应,合成4-芳基红藻氨酸类似物。
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves
其他客户在看
Anne Goldbech Olsen et al.
Nucleosides, nucleotides & nucleic acids, 22(5-8), 1331-1333 (2003-10-21)
To expand the triplex recognition repertoire of Nucleic Acids, novel nucleobases that recognize thymine in a T-A base pair are still required. A novel conformationally constrained PNA-monomer (II) capable of binding T in a triplex motif was designed and synthesized
Afzal Shah et al.
Bioelectrochemistry (Amsterdam, Netherlands), 77(2), 145-150 (2009-09-22)
The electrochemical behaviour of dimethyl-2-oxoglutarate (MOG), a key intermediate in the Krebs cycle and an important nitrogen transporter in the metabolic pathways in biological processes, was investigated by cyclic voltammetry, square wave voltammetry and differential pulse voltammetry using a glassy
Yuan Cao et al.
Theranostics, 7(12), 3021-3033 (2017-08-26)
Increased glutamine metabolism is a hallmark of cancer. Mitochondrial glutamic pyruvate transaminase (GPT2) catalyzes the reversible transamination between alanine and α-ketoglutarate (α-KG), also known as 2-oxoglutarate, to generate pyruvate and glutamate during cellular glutamine catabolism. However, the precise role of
An efficient synthesis of 4-aryl kainic acid analogs.
Maeda H, et al.
Tetrahedron, 55(4), 943-954 (1999)
Daniela Gaglio et al.
Molecular systems biology, 7, 523-523 (2011-08-19)
Oncogenes such as K-ras mediate cellular and metabolic transformation during tumorigenesis. To analyze K-Ras-dependent metabolic alterations, we employed ¹³C metabolic flux analysis (MFA), non-targeted tracer fate detection (NTFD) of ¹⁵N-labeled glutamine, and transcriptomic profiling in mouse fibroblast and human carcinoma
商品
This is an article about how proliferatively active cells require both a source of carbon and of nitrogen for the synthesis of macromolecules. Although a large proportion of tumor cells utilize aerobic glycolysis and shunt metabolites away from mitochondrial oxidative phosphorylation, many tumor cells exhibit increased mitochondrial activity.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门


![1,4-二叠氮双环[2.2.2]辛烷 ReagentPlus®, ≥99%](/deepweb/assets/sigmaaldrich/product/structures/366/129/a6ff4175-974d-4fac-9038-b35e508ef252/640/a6ff4175-974d-4fac-9038-b35e508ef252.png)