推荐产品
品質等級
化驗
95%
形狀
liquid
折射率
n20/D 1.504 (lit.)
bp
101-102 °C/12 mmHg (lit.)
密度
1.094 g/mL at 25 °C (lit.)
官能基
alkyl halide
bromo
儲存溫度
2-8°C
SMILES 字串
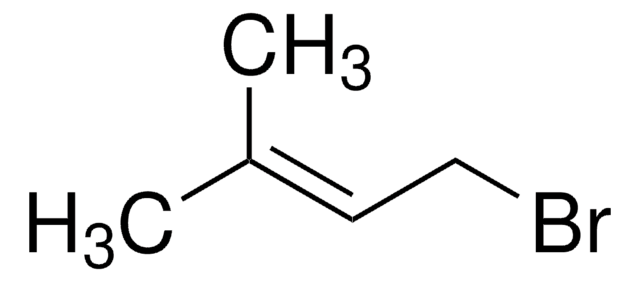
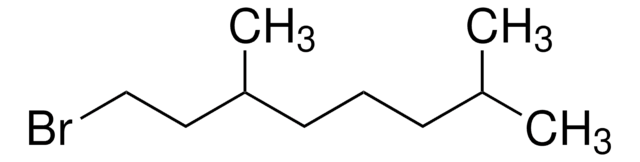
C\C(C)=C\CC\C(C)=C\CBr
InChI
1S/C10H17Br/c1-9(2)5-4-6-10(3)7-8-11/h5,7H,4,6,8H2,1-3H3/b10-7+
InChI 密鑰
JSCUZAYKVZXKQE-JXMROGBWSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
香叶基溴化物经钯催化,与芳基和链烯基金(I)磷杂发生交叉偶联反应。
應用
香叶基溴用于合成黄芩素和 3,7-二羟基黄酮衍生物。它还用于合成 P-糖蛋白活性的潜在黄酮类调节剂。
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
167.0 °F - closed cup
閃點(°C)
75 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
M Maitrejean et al.
Bioorganic & medicinal chemistry letters, 10(2), 157-160 (2000-02-15)
A new series of potential flavonoidic modulators of P-glycoprotein activity has been prepared. The flavanolignan silybin was first oxidised to dehydrosilybin and then C-alkylated with either prenyl or geranyl bromide. The resulting isoprenoid dehydrosilybins were shown to display high in
Marta Perro Neves et al.
European journal of medicinal chemistry, 46(6), 2562-2574 (2011-04-19)
Fourteen baicalein and 3,7-dihydroxyflavone derivatives were synthesized and evaluated for their inhibitory activity against the in vitro growth of three human tumor cell lines. The synthetic approaches were based on the reaction with prenyl or geranyl bromide in alkaline medium
Palladium-catalyzed cross-coupling reactions of organogold(I) phosphanes with allylic electrophiles.
Miguel Peña-López et al.
Organic & biomolecular chemistry, 10(8), 1686-1694 (2012-01-24)
Aryl and alkenylgold(I) phosphanes react regioselectively with allylic electrophiles such as cinnamyl and geranyl halides (bromide, chloride and acetates) under palladium catalysis in THF at 80 °C to afford the α-substitution product with moderate to high yields. When the reaction
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持